Fig. 1
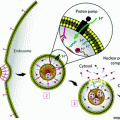
Illustration of the EPR effect depicting the discontinuous epithelium lining blood vessel walls of a tumor with concurrent poor lymphatic drainage compared to normal tissue. Nanoparticles can passively accumulate via gaps in blood vessels supplying tumor tissue. Adapted with permission from Ref. [4] Copyright (2007) Nature Publishing Group
In general, upon injection, nanoparticles can become coated with plasma proteins (opsonization), which mark them for rapid clearance by the mononuclear phagocyte system (MPS); the MPS is comprised of bone marrow progenitors, blood monocytes, and tissue macrophages (residing in both the liver and spleen) [6]. Since extended blood retention goes hand-in-hand with tumor accumulation, much research has been devoted to the development of “stealthy” long-circulating nanoparticles that avoid the MPS. To that end, there is a general set of guidelines for engineering long-circulating particles, which is depicted in Fig. 2. Briefly, particles should be either neutral or negatively charged, within a hydrodynamic diameter size range of 8–200 nm, and surface coated with a stealthing agent, such as polyetheylene glycol (PEG) [7]. Other particle parameters, such as shape and modulus, can also greatly influence the circulation profiles of nanomaterials [8–10], and other particle attributes can have highly variable effects. Targeting moieties and therapeutic cargo can alter in vivo behavior, including serum-biomolecule interactions, pharmacokinetic (PK) profiles, and biodistribution [11–13]. Inherent in the variability of all these properties is an optimal combination that may depend on the platform or fabrication technology. The interplay between particle parameters is further complicated in a biological setting, where the interaction between a nanoparticle and the immune system adds a significant layer of complexity. Ultimately, anecdotal evidence is not sufficient to predict the behavior of a given nanoparticle with complete confidence. Consequently, to increase the chance of successful translation, preclinical efforts should seek to understand particle behavior in the most relevant animal model available; traditional efficacy and toxicity assays should also be used.
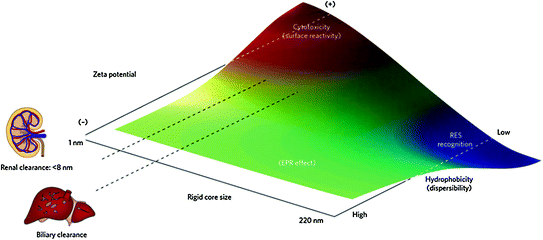

Fig. 2
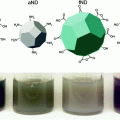
Qualitative trends in biological behavior and in vivo biocompatibility of nanoparticles based on their physical characteristics. Particles with neutral or negative surface charge, between 8 and 200 nm in size, and hydrophilic (well-dispersed) properties tend to promote enhanced permeation and retention (EPR) effects. Adapted with permission from Ref. [7], Copyright (2009) Nature Publishing Group
While many studies have investigated the behavior of particle systems in biological settings, revealing how particle properties work together presents unique challenges on the nanoscale. The major roadblock to reveal this interdependence is the previous lack of a suitable particle fabrication technique. Further, most studies on nanoparticles have focused on spherical shapes, due to their accessibility and narrow size distribution; such particles can be obtained by fabrication methods, such as dispersion, emulsion, and suspension polymerization [8, 14]. However, these systems lack sufficient control over size and shape. Particle replication in nonwetting templates (PRINT®) is one molding technique that allows for fabrication of “calibration quality” micro- and nanoparticles with independent control over their physical parameters (e.g., size, shape, surface chemistry, and modulus) [15]. The PRINT process is a continuous particle fabrication technology (Fig. 3) that begins by casting a film of pre-particle solution (this can vary from thermoplastic to thermoset materials) onto a high-surface energy delivery sheet (Fig. 3a), which is then laminated to the low-surface energy mold through a pressurized nip (Fig. 3b). Consequently, a reversible seal is formed between the mold and the substrate, allowing the liquid to be either confined to the cavities in the mold or forced out due to the low-surface energy of the interfacial area. Delamination of the mold from the delivery sheet results in PPS confined within the mold cavities, while excess PPS remains on the delivery sheet (Fig. 3c) The PRINT particles are then harvested from the mold as isolated, “scum-free” objects as depicted in Fig. 4. In this chapter, we will focus on the use of “calibration-quality” nanotools, such as PRINT-based particles, for studying the effect of various particle parameters—size, shape, modulus, drug loading, and surface chemistry—on the biodistribution, clearance, and delivery of therapeutics.
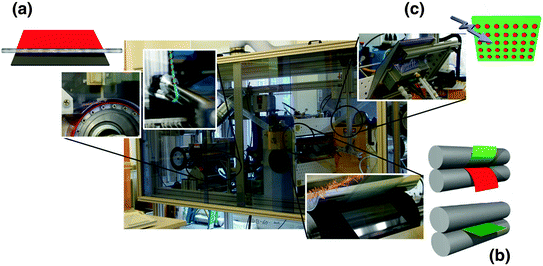
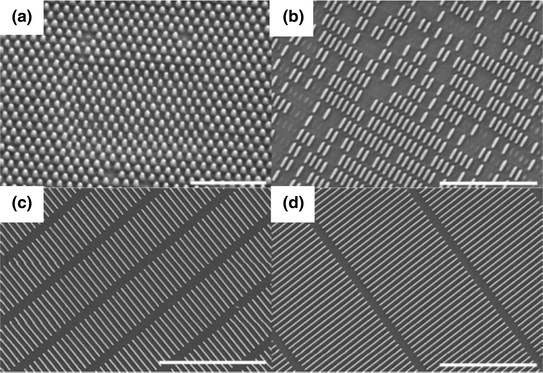

Fig. 3
Scale-up of the PRINT process to a roll-to-roll machine. PET and mold webs were mounted on the left side of the machine (unwind side), and threaded through various flexible unit operations before collecting on the right side (rewind side). a Film casting was achieved by corona treatment of the PET delivery sheet followed by film deposition by continuous dispensing of pre-particle solution (PPS) via a syringe pump behind a mayer rod. b Mold filling was accomplished by laminating the mold to the delivery sheet using a pressurized nip (heated for thermoplastics). The mold is then separated from the delivery sheet upon exiting the nip and the particles are formed within the mold either by c curing the PPS with a UV-LED oven (for thermosets) or by cooling the filled mold to room temperature (for thermoplastics)

Fig. 4
Scanning electron micrographs of PRINT particles fabricated from a 80 nm × 180 nm, b 80 nm × 320 nm, c 80 nm × 2000 nm, and d 80 nm × 5000 nm molds. Scale bar represents a 1 μm, b 3 μm, c and d 5 μm. Figure adapted with permission from Ref. [10], Copyright (2012) American Chemical Society
2 Size/Shape
Engineering a long-circulating nanoparticle for therapeutic delivery is absolutely paramount for tumor accumulation. In order to harness the EPR effect, particles need to stay in circulation long enough to passively accumulate at the target site (tumor) and then release their cargo. Size and shape are key parameters in designing a long-circulating particle. Much work has been done to lay the foundation for the appropriate particle size ranges; [16, 17] however, the body of work that has explored the effect of particle shape is smaller due to the limited availability of techniques to produce nonspherical particles. The recent advances in nanoparticle synthesis and fabrication technologies resulting in shape specific particles have started to elucidate the influence that particle shape can have over circulation profiles, biodistribution, and cellular entry kinetics once the particle has reached its target site [8, 18–25].
In terms of the particle circulation profiles, margination (defined as the movement of particles in flow toward the walls of the blood vessel) enhances the particles chances to extravasate through the leaky tumor vasculature and enter the tumor. Work—both empirical and theoretical—has been conducted in this area, comparing margination rates of spherical and nonspherical micro- and nanoparticles [20, 22, 26, 27]. In general, nonspherical particles with higher aspect ratios marginate more readily than spherical particles. Furthermore, high-aspect ratio particles were found to reduce macrophage uptake in vitro [19, 28, 29] translating to decreased liver and splenic filtration and increased circulation persistence of high-aspect ratio particles as compared to spherical particles [23, 28, 30].
To be effective, nanotherapeutics must avoid clearance by the liver, spleen, and macrophages. Once long circulation is achieved, particles need to accumulate at the target site. The passive accumulation of nanoparticles within tumors is contingent on their ability to extravasate through the leaky tumor vasculature, which is highly variable across tumor models. Even with this high degree of heterogeneity, there is a general consensus across literature that decreasing nanoparticle size can improve transport within tumors; [31–33] however, there is limited data concerning nonspherical particles. Recent work comparing nanospheres to nanorods of equivalent hydrodynamic size concluded that the nanorods could penetrate tumors four times as rapidly as nanospheres [34]. After particles have extravasated into the tumor bed, they then need to deliver their therapeutic cargo. Depending on the therapeutic, this may occur via extracellular or intracellular means (or a combination of both). If intracellular delivery is necessary, particle internalization can be enhanced either by modifying particle shape or incorporating active targeting to encourage receptor-mediated endocytosis (a topic discussed in a later section). Studies have shown that shape plays an important role in the way cells interact with particles. We have demonstrated that higher aspect ratio particles enhanced HeLa cell uptake kinetics in vitro [24], thus making it more likely for rod-shaped particles to be internalized by target cells.
Additionally, increasing the aspect ratio of the nanoparticle from 1 to 2.5 resulted in the more efficient delivery of chemotherapeutics to tumors. We evaluated the delivery properties of 200 nm × 200 nm and 80 nm × 320 nm PRINT poly (lactic-co-glycolic) acid (PLGA) nanoparticles loaded with similar concentrations of docetaxel [35]. Not surprisingly, both PRINT nanotherapeutics outperformed the small molecule clinical control, Taxotere. However, the higher aspect ratio 80 nm × 320 nm particles exhibited better PK profiles than the 200 nm × 200 nm particles [35]. Specifically, the rod-like particles exhibited enhanced blood and tumor retention with lower splenic and liver accumulation. This is translated to successful efficacy studies using the 80 nm × 320 nm geometry [36].
Studies by Discher and colleagues also clearly demonstrated the therapeutic benefits of using nonspherical drug delivery vehicles. They concluded that flexible, worm-like nanocarriers were more effective than spherical particles at delivering drugs and reducing tumor volume [37]. Work by Sailor et al. revealed similar results with spherical and high-aspect ratio magnetic particles and concluded that, when only one dimension was elongated, the size of the particles could be increased without sacrificing circulation times [38]. It is important to note that as aspect ratio increases, deformability becomes a crucial parameter to effectively navigate the biological barriers within the body [10, 14].
3 Modulus/Mechanical Properties
It is well documented that particles with a hydrodynamic diameter between 8 and 200 nm are large enough to avoid renal filtration and small enough to avoid liver and splenic filtration [7]. In addition to size and shape, the modulus of particles plays a role in their ability to avoid mechanical filtration by the liver and spleen, and has the ability to circumvent size limitations above the 200 nm cutoff. For instance, red blood cells are approximately 8 µm in diameter and, due to their extraordinary deformability, they can pass through splenic slits 2–3 µm in width [39]. However, as the red blood cells age and become stiffer, they can no longer deform and are removed from the blood stream by the spleen. We have developed polymeric, micron-sized, PRINT-based red blood cell mimics (RBCMs), whose mechanical properties can be controlled based upon their cross-link densities [40]. The biodistribution and circulation profiles of these particles were evaluated in vivo, and the results showed that the softer RBCMs were able to circulate longer than their stiffer counterparts [40]. Furthermore, at the nanoscale, we observed that PRINT-based, polymeric, 80 nm × 5000 nm filamentous particles that were fabricated with lower cross-link density were able to extravasate through 200 nm porous membranes that mimicked the pores found in the liver and spleen, whereas stiffer particles were unable to efficiently extravasate [10]. Extravasation of shorter filamentous particles (80 nm × 320 nm, and 80 nm × 180 nm) through the 200 nm porous membranes was independent of modulus [10].
Shaoyi Jiang’s lab reached similar conclusions in their investigations of the modulus of long-circulating zwitterionic spherical nanoparticles. Spherical particles (d = 250 nm) with a range of cross-link densities were fabricated and were passed through 220 nm porous membranes [41]. While all the “soft” particles were able to pass through the filter, the majority of the “hard” particles (with the highest cross-link densities) were stopped by the filter [41]. These trends were also predictive of in vivo particle behavior. As the cross-linker density of the particle was decreased, splenic accumulation was minimized, resulting in extended circulation persistence [41]. With a better understanding of the relevant particle parameters necessary for extending particle circulation, the focus shifts to drug loading and release.
4 Drug Loading/Drug Release Rates
Promising drug candidates are often hydrophobic compounds with poor aqueous solubility. To increase their solubility, they are typically formulated in mixtures of low molecular weight surfactants and organic solvents, which have their own nonspecific toxicity. Particulate formulations of these drugs can not only eliminate the necessity of organic solvents and surfactants, but can also alter the pharmacokinetics of the delivered therapeutic. Since small molecule drugs can extravasate through the tight endothelial junctions lining the blood vessel walls, they can freely permeate throughout the body, interacting with both healthy and diseased tissue indiscriminately and resulting in dose-limiting systemic toxicities. Formulating these drugs within a nanoparticle can keep the drug within the blood compartment (reducing the volume of distribution and interactions with healthy tissues) and allow a greater portion of the injected dose to potentially reach the target cancer cells. Maintaining controlled release of the drug from the nanotherapeutic platform is also crucial for improved efficacy and reduced toxicity. Ideally, the carrier should allow minimal premature release of drug before it reaches the intended site, but sufficient release after it reaches the target. Drug release from the particle carrier can be dictated by particle erosion and diffusion (as with biodegradable particle matrices) or by external (including light, ultrasound, and magnetic fields) or physiological (pH gradients and redox states) stimuli [42–46].
Biodegradable PLGA (poly lactic-co-glycolic acid), a thermoplastic material, is one example of a noncovalent entrapment system that is commonly used in nanoformulations [47]. For this particle matrix, generally hydrophobic drugs are noncovalently loaded by physical entrapment or weak intermolecular interactions, and release rates are based upon drug loading, matrix degradation, and passive diffusion. Typically, these particles suffer from an immediate burst release, followed by sustained drug release [47]. Polymer or lipid coatings can be used to overcome this burst release profile. Sethi et al. utilized a cross-linkable lipid shell to decrease the release rate of wortmannin and docetaxel from PLGA particles [48]. The slower release rate translated to enhanced efficacy; while nanoparticles without a cross-linked lipid shell showed no benefit over the free drug [48].
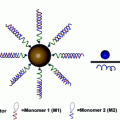
Another method to alter drug release from the particle is to modify the drugs’ affinity to the particle matrix. This can be done through a prodrug approach. Prodrugs are derivatives of drugs that undergo stimuli responsive triggering to release the active drug, offering protection from inactivation and toxic side effects [49]. Using this approach, docetaxel was lipidated through an acid-sensitive, silyl ether linkage and encapsulated within a PRINT-based PLGA nanoparticle (PRINT-C2), depicted in Fig. 5a [50]. The addition of the lipid tale enabled drug release to be extended from 24 h (for the unmodified drug) to 96 h (Fig. 5c). Furthermore, once the prodrug was released from the particles, it underwent complete conversion to the active form of the drug in plasma after 24 h. PK analysis showed that the PRINT-C2 nanoparticle had greater persistence in the plasma than both PRINT-docetaxel and Taxotere, and this can most likely be attributed to the slower release rate from the nanoparticle. A significant finding of this study was that the prodrug preferentially converted to the active drug in the tumor, most likely due to the acidic nature of the tumor tissue as compared to healthy tissue [51]. This adds another layer of control for mitigating unwanted side effects.
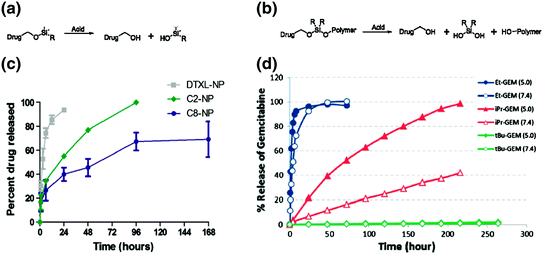

Fig. 5
Prodrug approaches for incorporating drugs into nanoparticles. Two types of silyl ether prodrugs, a small molecule monofunctional silyl ether and b polymeric asymmetric bifunctional silyl ether prodrug, for encapsulation into PRINT PLGA or PEG hydrogel particles, respectively. c Release kinetics of PLGA particles loaded with unmodified Docetaxel (DTXL-NP) or monfunctional silyl ether prodrugs with R equal to ethyl (C2-NP) or octyl (C8-NP). d Release kinetics of hydrogel particles loaded with bifunctional silyl ether Gemcitabine, with either to ethyl, isopropyl, or t-butyl R groups. Figures adapted with permission from Refs. [50] and [52], Copyright (2012 and 2014) American Chemical Society
Encapsulation, however, is poorly suited to intrinsically porous lowly cross-linked hydrogel particles. For drugs that are difficult to retain in these types of particles we have developed a silyl ether-based prodrug approach that utilizes covalent linkages, depicted in Fig. 5b [52]. The prodrug contains an acrylate group and can therefore be directly polymerized into thermoset particles. The drug release kinetics were found to be pH dependent, and the rates could be controlled by the bulkiness of the silicon substituents (Fig. 5d). In vitro experiments demonstrated that particles could be fabricated to release drugs, such as gemcitabine and camptothecin, with comparable toxicities to the free drug. Using a similar approach, Wang et al., developed a pH sensitive gemcitabine-poly(methyl methacrylate) prodrug (GEM-PMAA), which self-assembles in water to form nanoparticles [53]. Drug release rates were found to be pH dependent and in vivo assessment proved that the particles could efficiently inhibit tumor growth and alleviate drug-associated side effects [53]. Drug release from these pH responsive linkers can occur in the acidic conditions of the tumor tissue or intracellular compartments. If intracellular delivery is necessary, nanocarriers can be modified with targeting ligands to improve cellular internalization.
5 Surface Functionalization for Passive and Active Targeting
Current methods of nanoparticle delivery to tumors are based mainly upon either passive or active targeting. Passively targeted particles are typically surface functionalized with stealthing agents, to allow for long circulation and thus accumulation through the EPR effect. Actively targeted particles are modified with targeting ligands used to recognize overexpressed receptors on the tumor cell surface. For either delivery mechanism, a common feature is that the nanoparticles in the bloodstream must first marginate and then extravasate into the interstitium of the tumor. Passive targeting relies heavily on extending particle circulation half-life, thus increasing their chances of reaching their target site. This is commonly achieved by conjugating, grafting, or adsorbing polyethylene glycol (PEG) to the surface of nanoparticles (PEGylation). Altogether, effects of PEGylation are highly dependent on two interrelated parameters: the molecular weight (MW) and surface density of the PEG coating. In general, PEG ranging from 2 to 10 k displays adequate resistance to protein adsorption and subsequent phagocytic uptake [54]. The density of PEG necessary to promote protein resistance and extend blood circulation varies drastically for different nanoparticle types. Generally, metallic and other highly immunogenic particle types require significantly greater surface PEG densities as compared to more inert matrices [55]. We have demonstrated that our cross-linked PEG matrix required a much lower surface density of PEG (less than 0.1 PEG molecules per nm2) to enhance protein resistance and extend circulation half-life (from 0.89 to 19.5 h) [55]. Other polymers for surface passivation, such as zwitterionic coatings and sugar-based moieties, have shown promise [56, 57]. While passivation remains the most utilized surface modification, active targeting has become a fixture in particle platforms. The “holy grail” in drug delivery is to create a system that can be used to deliver a highly potent therapeutic to the diseased, cancerous tissue, while completely eliminating exposure to off-target, healthy cells [58]. To achieve targeted drug delivery, nanoparticles can be coated with ligands that bind specifically to particular overexpressed receptors on the diseased cell surface. Commonly targeted receptors implicated in various cancers include the epidermal growth factor receptor (EGFR), the vascular endothelial growth factor (VEGF), and the human epidermal growth factor receptor (HER-2, in the EGFR family), to name only a few [11, 59]. By designing a drug carrier that targets these receptors, preferential cell interactions can occur with the diseased tissue, leading to reduced exposure in healthy tissue. In reality, off-targeting remains since target-receptor expression in healthy cells persists, albeit to a lesser extent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree