html xmlns=”http://www.w3.org/1999/xhtml”>
52
Cancer Immunotherapy with Vaccines and Checkpoint Blockade
Therapeutic vaccination for cancer continues to be a major approach to the overall immunotherapy of cancer. Historically, interest in cancer immunology stemmed from the perceived potential activity of the immune system as a weapon against cancer cells. In fact, the term magic bullet, commonly used to describe many visions of cancer therapy, was coined by Paul Ehrlich in the late 1800s in reference to antibodies targeting both microbes and tumors. Central to the concept of successful cancer immunotherapy are the dual tenets that tumor cells express an antigenic profile distinct from their normal cellular counterparts and that the immune system is capable of recognizing these antigenic differences. Support for this notion originally came from animal models of carcinogen-induced cancer in which it was demonstrated that a significant number of experimentally induced tumors could be rejected on transplantation into syngeneic immunocompetent animals. 1–4 Extensive studies by Prehn on the phenomenon of tumor rejection suggested that the most potent tumor rejection antigens were unique to the individual tumor. 5
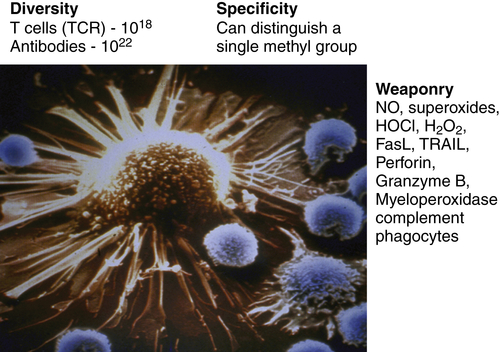
Figure 52-1 The immune system as the perfect anti-cancer weapon Shown in the figure is a single cytotoxic T lymphocyte specific for a tumor antigen expressed by the tumor cell it is about to kill. Although other lymphocytes contact the tumor cell, if they do not express a T-cell receptor specific for a peptide derived from a protein in the tumor cell and presented on MHC class I, they will ignore the tumor cell. Fundamentally, the immune system is endowed with all of the assets desired to specifically eliminate cancer cells while having a minimal effect on normal cells. The tremendous diversity of T-cell receptors and antibodies affords the adaptive immune system both specificity and adaptability. The MHC transport system that carries peptides from degraded proteins to the cell surface allows T cells to recognize protein antigens expressed anywhere in the cell. In addition, the immune system can produce more than 20 cytocidal molecules with diverse mechanisms of killing once activated.
Since the original reports of Jenner over two centuries ago, prophylactic vaccination against infectious diseases has been one of the most influential medical interventions. Cancer vaccination, an immunotherapy approach applied to patients with established cancer, has tremendous potential based on the ability of both T cells and antibodies to specifically recognize cancer antigens and kill cancer cells expressing these antigens. However, at the time of this writing, only one human cancer vaccine has received U.S. Food and Drug Administration (FDA) approval, despite multiple Phase III clinical trials over the past two decades. Despite the clinical failures of cancer vaccines to date, continuing molecular definition of tumor-specific and tumor-selective antigens, new vaccine platforms that selectively target and activate dendritic cells, and preclinical results with combinations of vaccination together with other immune modulators have generated renewed optimism that cancer vaccination will ultimately take its place among the pantheon of cancer therapies.
As cancer genetics and genomics have exploded over the past decade, it is now quite clear that altered genetic and epigenetic features of tumor cells indeed result in a distinct tumor antigen profile. Overexpression of “oncogenic” growth factor receptor tyrosine kinases such as HER2/Neu and epidermal growth factor receptor (EGFR) via epigenetic mechanisms has provided clinically relevant targets for one arm of the immune system—antibodies. 6,7 Indeed, monoclonal antibodies are the fastest growing single class of cancer therapeutics based on successful new FDA approvals. In striking contrast, cellular immunotherapy of cancer has been quite disappointing in establishing therapeutic success in clinical trials to date. Emerging insights about the nature of the interaction between the cancer and the immune system have led us to understand why cell-based cancer immunotherapy approaches such as therapeutic vaccines have been less potent against established cancer than originally imagined. In general, we have learned that tumors employ mechanisms of tolerance induction to turn off T cells specific for tumor-associated antigens. Oncogenic pathways in tumors result in the elaboration of factors that organize the tumor microenvironment in ways that are quite hostile to antitumor immune responses.
Not only is the cancer capable of inducing potent tolerance among tumor-specific T cells, we now know that there are distinct forms of inflammatory and immune responses that are procarcinogenic. Thus, two frontiers in cancer immunology are the elucidation of how the tumor organizes its immune microenvironment and the nature of immune responses that are anticarcinogenic versus procarcinogenic. As the receptors, ligands, and signaling pathways that mediate immune tolerance and immune-induced procarcinogenic events are elucidated, these factors and pathways can be selectively inhibited by both antibodies and drugs in a way to shift the balance to antitumor immune responses. This chapter outlines the major features of tumor–immune system interactions and set the stage for molecularly based approaches to manipulate immune responses for successful cancer therapy. The clinical results over the past few years, particularly with checkpoint blockade, validate clinically the tremendous potential of the immune system to destroy cancer cells (Figure 52-1 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree