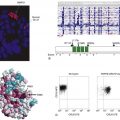
Targeting the PI3K/Akt/mTOR signaling pathway is another promising approach, especially in the lung. Tobacco carcinogens induce Akt activation and lung carcinogenesis. The Akt pathway is activated in bronchial premalignancy (both proximal airway and alveolar epithelium) in smokers and patients with lung IEN or cancer. Preclinical in vivo studies show that deguelin and myo-inositol have preventive activity in lung tumorigenesis, in part via suppressing the PI3K/Akt pathway, disrupting Hsp90 function, and inhibiting HIF-1α expression.
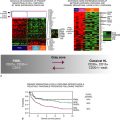
64–66 The kinase mammalian target of rapamycin (mTOR) is downstream of Akt, and the mTOR inhibitor CCI-779 blocked malignant progression of premalignant lesions with activated mTOR arising in the alveoli of mice that develop lung cancer because of activated K-ras.
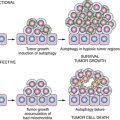
67 The mechanism by which CCI-779 inhibited tumorigenesis was unexpected. These lesions were infiltrated with macrophages, shown immunohistochemically to have prominent activation of mTOR signaling. A similar pattern of macrophage infiltration occurred in human alveolar premalignant lesions (atypical alveolar hyperplasia). Treatment with CCI-779 induced apoptosis of macrophages, which coincided with the chemopreventive effect. In vitro, CCI-779 had no effect on LKR-13, a lung adenocarcinoma cell line derived from this mouse, whereas it did induce apoptosis of macrophages, and conditioned media from macrophages directly stimulated the proliferation of LKR-13 cells. In summary, mTOR is activated in lung premalignancy and is required for malignant progression in the lung. This kinase drives tumorigenesis in part through macrophages, a prominent component of the tumor microenvironment, and the antitumor effect of mTOR inhibition required the presence of the tumor microenvironment. These findings have two important implications: mTOR is a potentially important kinase target, and the tumor microenvironment is crucial in malignant progression and a source for novel targets in chemoprevention. An mTOR inhibitor also has reversed Akt-dependent prostatic IEN in transgenic mice.
68