Introduction
The risk of most types of cancer increases with age and with the growth in the aged segment of the population, the burden of cancer in the elderly will continue to grow. In this chapter, the scope of this problem is reviewed, as is the biology of cancer and ageing. A discussion on cancer prevention and treatment in the elderly follows. Finally, supportive care, survivorship issues and the multidisciplinary care of the senior adult cancer patient are reviewed.
Epidemiology and Disparities
Cancer is the leading cause of death in men and women aged 60–79 years and the second leading cause of death in persons aged 80 years and older.1 By 2030, more than one-fifth of the population in the USA will be over the age of 65 years.2 The probability of developing cancer is one in three in men and one in four women over the age 70 years. The leading causes of cancer incidence and mortality are detailed in Figure 108.1.
Figure 108.1 (a) Cancer incidence rates in men; (b) cancer incidence rates in women; (c) cancer mortality rates in men; (d) cancer mortality rates in women. All rates age adjusted to 2000 US population. 2006 SEER statistics.
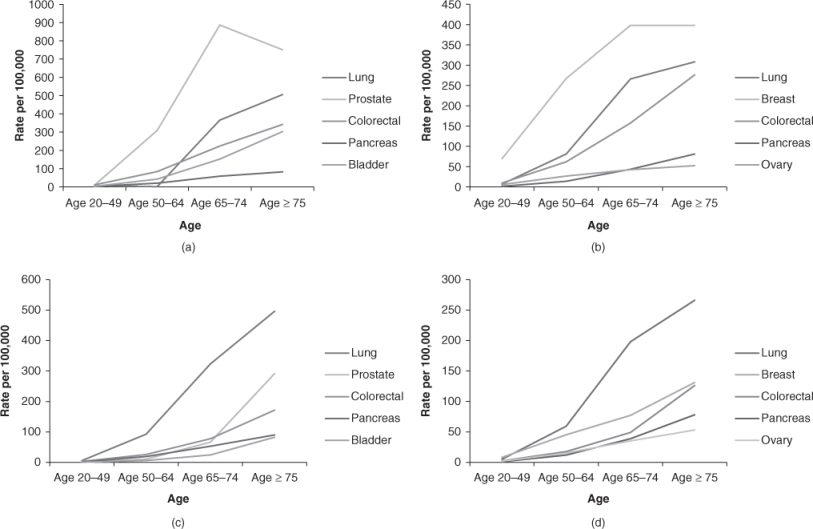
Over the past 60 years, cancer-specific death rates have decreased among younger individuals, while increasing in older individuals.3 Significant disparities in outcomes between younger and older individuals are likely due to a number of factors, including differences in screening, more advanced stage at presentation in older individuals or less aggressive treatment in older patients. Older individuals are more likely to experience delays in diagnosis, incomplete evaluation and undertreatment. Half of older women receive substandard treatment for breast cancer, with significantly worse survival.4 Similar trends have also been noted among patients with ovarian and rectal cancer and these persist even when studies control for comorbidities and functional status. Under-enrolment of older individuals in clinical trials further compounds the situation by resulting in a paucity of data on appropriate management of cancer in the elderly.
Ageing and Tumour Development
Hanahan and Weinberg proposed that there are six attributes that must be attained by a cell to be transformed into a malignant cell: self-sufficiency in growth signals, insensitivity to anti-growth signals, evasion of programmed cell death, limitless replicative potential, sustained angiogenesis and tissue invasion/metastasis.5 Mutations cause cellular changes resulting in these altered characteristics and in malignant transformation.
Theories of biological ageing and carcinogenesis overlap in many ways, potentially explaining the increased incidence of many cancers with age. Over time, DNA damage caused by random events or free radicals can cause either cellular dysfunction/death, resulting in ageing, or may cause mutations in proto-oncogenes or tumour suppressors, yielding carcinogenesis. Further, changes seen in cells with ageing are also observed in early carcinogenesis. The formation of DNA adducts, DNA hypomethylation, chromosomal breakage and translocation are associated with age and increase the susceptibility to late-stage carcinogens.6
The immune dysregulation associated with ageing may contribute to the increased incidence of cancer with age. With age, changes in T-cell function result in decreased proliferation, increased proportion of memory cells and a decrease in naive T-cells. B-cell function is intact but dysregulated, with an increase in autoantibody formation and monoclonal protein production. Interleukin-2 levels decrease, whereas interleukin-6 levels rise. A prospective cohort study demonstrated that individuals with better NK cell function had lower rates of cancer 10 years subsequently.6
In some ways, however, ageing and cancer biology are at odds: cancer requires limitless replicative potential, while finite replicative potential (replicative senescence) is a hallmark of ageing. Most normal human cell types have the capacity for 60–70 doublings. The cellular ‘abacus’ is the telomere, which consists of several thousand repeats of a short base pair sequence at the ends of every chromosome. The telomeres protect the chromosomal DNA. With each successive replication, 50–100 base pairs of telomeric DNA are lost from the ends of the chromosomes. Over time, in normal cells, these protective caps are lost; the chromosomal DNA becomes fused end-to-end with other chromosomes, ultimately leading to death of the affected cell. In contrast, in malignant cells, telomeres are maintained through the expression of telomerase, allowing unlimited replication.7 Another mechanism of senescence, termed ‘stress-induced premature senescence’, results from cellular events other than telomere shortening. Mutations in an oncogene or double-stranded DNA breaks induced by chemotherapeutic agents can trigger senescence, resulting in a proportion of clonal cells entering senescence. This permanent growth arrest may be as effective as apoptosis as an anti-cancer mechanism.8
In some malignancies, there are age-related differences in tumour biology, making the malignancy either more or less aggressive in older patients compared with their younger counterparts. It is a commonly held, though debatable, dogma that solid tumours, including breast, colon, lung and prostate cancer, are more indolent in older patients; however, epidemiological data do not altogether support this observation. It is clear that in some cancers, there are differences in tumour behaviour over the age spectrum. Breast cancers in older women are more likely to be estrogen receptor positive. Acute myeloid leukaemia is more aggressive in elderly patients and more resistant to conventional chemotherapy due to the increased expression of the MDR1 (multidrug resistance) gene.
Cancer Prevention
Cancer prevention is an effective way to reduce cancer morbidity and mortality. Cancer prevention strategies include behavioural/lifestyle modification, such as dietary changes, chemoprevention and screening.
Obesity is associated with post-menopausal breast cancer and weight loss lowers circulating estrogen levels. Large cohort studies demonstrate that weight loss is associated with a decreased risk of post-menopausal breast cancer.9 Further, a randomized trial of a lower fat dietary intervention, which resulted in weight loss in the intervention group, was associated with an 11% reduction (hazard ratio 0.89, 95% confidence interval 0.80–1.00) in estrogen receptor positive (ER+) post-menopausal breast cancer diagnoses in the 8 years of follow-up.10 Further research is needed into whether it is weight loss per se or dietary modification that results in the reduced risk of cancer. In this same trial, however, there was no change in the incidence of colorectal cancer with the dietary intervention.11
Epidemiological studies suggest a protective effect of increased calcium and vitamin D intake on the risk of colorectal cancer. Randomized trials have demonstrated a modest but significant decrease in risk of recurrent adenomas. In the Women’s Health Initiative randomized trial, supplementation with calcium and vitamin D did not result in a decrease in the risk of colorectal cancer versus placebo.12 However, this study was criticized for doses of vitamin D3 (400 IU daily) that are generally inadequate to achieve sufficient serum levels of 25-hydroxyvitamin D.
The inducible enzyme cyclooxygenase 2 (COX-2) is elevated in the majority of colorectal cancers. Aspirin, a non-specific inhibitor of both COX-1 and COX-2, reduces the risk of colorectal cancer by 24% in patients who take at least 300 mg of the medication for at least 5 years and after a latency period of 10 years.13 However, the benefit of cancer prevention must be weighed against the risk of bleeding complications. The COX-2 inhibitor’s more selective mechanism results in lower risk of bleeding. Indeed, the COX-2 inhibitors rofecoxib and celecoxib effectively prevent the formation of precancerous polyps, but are also associated with an increased risk of cardiovascular events.14 Given the increased risk and no data showing a decreased risk of invasive colorectal cancer, COX-2 inhibitors should not yet be used for colorectal cancer prevention.
The selective estrogen receptor modulators (SERMs) compete with estrogen for binding at the estrogen receptor, inhibiting pathways required for cellular growth and proliferation. A randomized, placebo-controlled trial of tamoxifen for the prevention of breast cancer enrolled over 13 000 women. Tamoxifen reduced the risk of invasive breast cancer by more than 40%. Therapy with tamoxifen was associated with a twofold increased risk of pulmonary embolism and a threefold increased risk of endometrial cancer.15 Given the serious potential side effects, tamoxifen is recommended only for women at high risk for cancer using risk prediction models such as the Gail model, weighed against the individual risk factors for adverse events.16
Another SERM, raloxifene, has been studied with regard to its impact on the incidence of post-menopausal breast cancer. Over the 8 years of follow-up, therapy with raloxifene was associated with a 76% reduction in the incidence of ER+ breast cancers, relative to placebo. Women treated with raloxifene had a twofold increased risk of venous thromboembolic disease.17 In a head-to-head comparison, postmenopausal women at increased risk for breast cancer were randomized to either raloxifene or tamoxifen. Raloxifene was as effective as tamoxifen at reducing the risk of invasive breast cancer, with a lower risk of venous thromboembolic events.18
In summary, several cancer prevention strategies hold promise. However, in an individual patient, the risks and benefits must be considered before recommending chemopreventive strategies.
Cancer Screening
Screening as a strategy for prevention is a complicated issue. The goal of screening is to identify a disease during a latent or early symptomatic stage, in order to intervene and alter the natural history of disease. Although there is evidence of benefit of screening for breast, colorectal and cervical cancer in individuals in their 50s and 60s, data for screening older, asymptomatic individuals for these common malignancies is lacking. Complicating the issue are the differences in cancer biology described above, which may result in the detection of more indolent cancers that would not ultimately be life-limiting. The sensitivity and specificity of screening tests may be affected by age-related changes in body composition, such as the change in breast composition with age. The harm due to false-positive screening tests must also be taken into account, including psychological distress and risks of diagnostic procedures. Comorbid medical conditions and functional decline may increase the risk of complications related to screening procedures, such as the sedation required for colonoscopy. Table 108.1 gives a summary of current recommendations regarding screening.
Table 108.1 Recommendations for cancer screening in older adultsa.
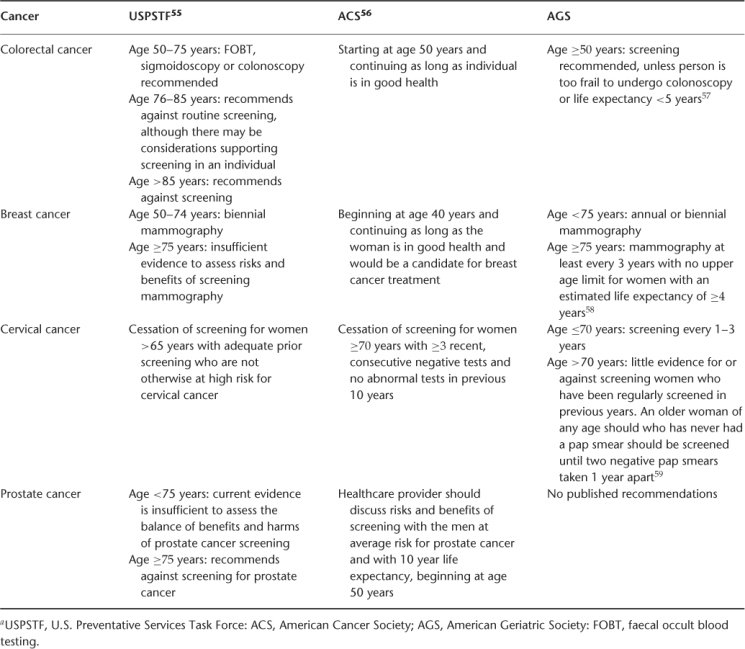
Colorectal cancer screening is effective in selected older patients. In patients aged 70–80 years, randomized trials have shown annual to biennial faecal occult blood testing (FOBT) to be effective at reducing colorectal cancer incidence and mortality, with a lag time of 5 years to mortality benefit. However, the sensitivity and specificity are low. Case–control studies of flexible sigmoidoscopy and colonoscopy in older patients suggest a mortality benefit associated with screening; however, sigmoidoscopy has a lower sensitivity in older individuals given the increase in prevalence of right-sided colon cancers, which sigmoidoscopy does not detect. Colonoscopy is more sensitive and specific than FOBT or sigmoidoscopy, but in a cohort of older patients aged 70–75 years, the rate of major complications of colonoscopy, including perforation, myocardial infarction or stroke, was 0.3%.19 Computed tomographic (CT) colonography appears to be as effective as colonoscopy in older patients at detecting advanced neoplasias, with a low false-positive rate,20 but must be followed by traditional colonoscopy to confirm abnormal findings.
Among the randomized trials that established the mortality benefit of mammographic screening for breast cancer, only one included women over the age of 70 years. Although in the overall study mammography decreased the risk of breast cancer death by one-third, there was not a significant reduction in breast cancer mortality in the subgroup of women aged 70–74 years. Interestingly, as the density of breast tissue decreases with age, the sensitivity and specificity of mammography increase. There are several studies demonstrating that among women with multiple comorbidities, detecting early-stage breast cancer does not improve survival. Thus, women with a life expectancy of less than 5 years are unlikely to benefit from breast cancer screening.19
There have been no prospective randomized trials of cervical cancer screening in any age group, although multiple observational studies show that screening with Papanicolaou (Pap) smears decreases the incidence and mortality of cervical cancer. With advancing age, the sensitivity of Pap smears decreases due to the migration of the squamo-columnar junction into the cervical canal and specificity declines due to atropic changes causing inflammation. Older women who have been regularly screened are at low risk and those who have significant comorbidity are unlikely to benefit from screening.19
Prostate cancer screening is controversial, even in younger populations. Prospective, randomized trials of prostate-specific antigen (PSA) screening are under way, but do not include men over the age of 75 years. Given the indolent nature of most prostate cancers and high prevalence of clinically irrelevant prostate cancers found among octogenarians at autopsy, screening for prostate cancer is generally not recommended in men over the age of 75 years or who have a life expectancy of less than 10 years.21
In summary, selected older individuals with longer life expectancy may be appropriate for screening for common cancers.
Cancer Treatment
The basic tenets of cancer treatment involve the determination of whether treatment is being undertaken with curative or palliative intent. Radiation therapy and surgery are the modalities of treatment utilized to control the local extent of cancer. Cytotoxic chemotherapy, hormonal therapy, biological therapy or targeted agents are typically administered orally or intravenously for systemic treatment. Treatment may be administered neoadjuvantly, that is, prior to definitive treatment, to limit the chance of systemic spread and to decrease the extent of local treatment. Adjuvant therapy is administered following definitive treatment, to reduce the risk of recurrence in individuals at high risk. Palliative treatment is administered to improve symptoms or prolong life in patients with an incurable malignancy.
Decision-Making
Historically, clinical trials of cancer treatment have excluded patients due to advancing age or therapies in older patients. Clinicians have been left to extrapolate from data derived from the treatment of younger individuals. Increasing attention to this problem has yielded both retrospective and prospective studies of the effectiveness of treatment strategies in older adults with cancer, but much work remains to be done. Guidelines for the approach to management of cancer in senior adults have been established (Figure 108.2).
Figure 108.2 Guideline for management of senior adult oncology patients. Adapted with permission from the NCCN 1.2010 Senior Adult Oncology Clinical Practice Guidelines in Oncology. ©National Comprehensive Cancer Network, 2010. The most recent and complete version of the guideline is available at http://www.nccn.org.

Surgery
Surgery is employed in the treatment of cancer to remove the primary tumour with curative intent. Surgery may also be used to palliate symptoms or prevent serious complications in advanced or metastatic disease, such as colonic diversion for an obstructing colon mass. While some studies have been concerned with increased risk of adverse outcomes in older individuals, often these did not account for comorbidities, advanced cancer stage at presentation, functional impairment and other confounding factors.22 Appropriate preoperative risk stratification must be employed. A tool developed specifically for use in older cancer patients, the Preoperative Assessment of Cancer in the Elderly (PACE), incorporates measures of cognition, functional status, depression, fatigue, ECOG performance status, American Society for Anesthesiology scale (ASA) and Satariano’s index of comorbidities. In a prospective international study of patient 70 years of age and older undergoing elective cancer surgery for solid tumours under general anaesthesia, 460 patients underwent this oncogeriatric-specific assessment on the day prior to surgery. Any dependencies in instrumental activities of daily living (IADLs), moderate to severe fatigue or abnormal performance status were associated with a 50% increased risk of 30 day morbidity following surgery. Similarly, any dependencies in ADLs or IADLs and abnormal performance status were associated with a longer than expected hospital stay; dependency in ADLs doubled the risk of an extended postoperative hospital stay.23 Interestingly, in this study, comorbidities did not independently predict postoperative complications. Overall, older adults with cancer should not be excluded from surgery on the basis of age alone, but the decision to proceed should be individualized based on the risks of the procedure, potential benefits, the patient’s functional status and goals of care.
Radiation
Radiation therapy can be employed with either curative or palliative intent. Some tumours, such as stage I lung cancers or localized prostate cancer, can be effectively treated with radiation alone. Radiation may also be used in conjunction with surgery to improve local control of cancer, as in post-lumpectomy breast irradiation for breast cancer or preoperative radiation for rectal cancer. Radiation may also provide palliation of symptoms in patients with advanced malignancies. Techniques for the delivery of radiation therapy, utilizing three-dimensional imaging reconstruction, have evolved dramatically recently, allowing improved tolerability with sparing of normal tissues. These include conformal radiation, intensity-modulated radiation and stereotactic radiation therapy. Brachytherapy involves the placement of a radioactive source directly within the site of tumour cells and is used in prostate, breast, cervical and skin cancer. Radiation may be delivered systemically in a few instances. Bone-targeting radioisotopes such as samarium-153 and strontium-89 localize to areas of osteoblastic lesions and are helpful in treating painful osteoblastic bone metastases. Radioimmunotherapy consists of radioactive isotopes conjugated to monoclonal antibodies. Examples of radioimmunotherapy include ibritumomab and tositumomab, which are used in non-Hodgkin lymphomas, target the B-cell marker CD20 and are safe and effective in older patients.
In general, there is no decrement in the benefit of radiation therapy in older compared with younger patients and no increase in toxicity in the elderly. Rectal cancer and malignant gliomas are exceptions to this statement. In older women with high-risk breast cancer, post-lumpectomy radiation improves survival. Elderly prostate cancer patients receiving radiation for localized disease have similar 10 year survival rates and disease-free survival rates compared with younger patients and should not be denied potentially curative therapy based on age alone.24 Toxicities in the elderly are similar to those in younger patients, although older patients tend to have more functional decline acutely during therapy. In subset analyses of combination chemoradiation for lung cancer, patients over the age of 70 years tended to have more frequent oesophagitis and neutropenia, but still enjoyed a survival benefit without increased long-term complications.25 Modern radiation techniques can also minimize long-term consequences of therapy, such as sparing the contralateral salivary glands in head and neck radiation to prevent xerostomia.
The toxicity of radiation to the brain in older patients and its effect on cognition warrant specific discussion. Acute reactions occur during treatment, are associated with cerebral oedema and can be controlled with corticosteroids. Early delayed reactions occur weeks to months after completion of radiotherapy and are characterized by somnolence and lethargy. This is associated with demyelination, endothelial damage, small-vessel thrombosis, accelerated atherosclerosis and long-term memory deficits. Late delayed injuries
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








