CALCIUM STONES
Part of “CHAPTER 69 – NEPHROLITHIASIS“
PATHOGENETIC FACTORS
The factors critical to calcium stone formation are supersaturation and nucleation. Supersaturation of a solution occurs when a solution contains a salt (e.g., calcium oxalate) at a concentration that exceeds its solubility.1,8 When such a solution comes into contact with a precipitated salt with which it is already supersaturated, the solid phase serves as a nucleation center for the aggregation and precipitation of the excess salt in solution. Supersaturation is defined and measured in terms of the particular salts present in concentrations exceeding their solubilities. A solution may be supersaturated with several salts, each to a different extent. The magnitude of the supersaturation provides the driving force for crystallization of the solid phase (e.g., stone formation from urine).
The supersaturation of urine with calcium oxalate and calcium phosphate phases, such as brushite or apatite, is determined by (1) the concentrations of calcium, oxalate, and phosphate; (2) the concentrations of calcium ligands such as citrate (which chelates calcium, preventing the precipitation of calcium oxalate or phosphate salts); and (3) the pH (determines relative concentrations of hydrogen phosphate and phosphates, which form brushite and apatite, respectively). The actual measurement of supersaturation is difficult but useful, because the elevation of supersaturation corresponds to stone composition.9 Clinically, the excretion rates and concentrations of calcium and oxalate and the urine pH are measured to detect patients with hypercalciuria, hyperoxaluria, or abnormally alkaline urine. These measurements frequently represent the diagnostic end points of patient evaluation for supersaturation.
Important to the formation of renal stones is a surface (nucleation center) within the supersaturated solution that can promote aggregation of ions to form crystals. Given a foreign surface to which ions can adhere to form clusters, stones can be formed, even if supersaturation is mild; therefore, the probability of stone formation can be increased simply by a foreign nucleation surface. Clinical examples of foreign, or “heterogeneous,” nucleation include stone crystallization on an injured urothelial surface and calcium oxalate stones grown on a crystal of uric acid induced by low urine pH and increased uric acid excretion. The uric acid crystals offer a nucleation surface on which calcium oxalate overgrowth is likely to occur.10 When the foreign surface strongly resembles the one to be formed, the ions aggregate on the surface in a strongly oriented manner, reflecting the alignment of the overgrowing and preformed crystals. This process is called epitaxial growth, and although it has been proposed as a mechanism for uric acid nucleation of calcium oxalate,10,11 it is unlikely to be a common occurrence.
Urine contains substances that inhibit the growth and formation of calcium oxalate and phosphate stones. Citrate, which lowers the supersaturation of calcium salts by chelating calcium, can also reduce the growth of calcium oxalate and phosphate crystals.12,13 An anionic glycoprotein inhibi- tor, glycoprotein crystal growth-inhibitor (GCI) protein,14,15 which is in urine, dramatically inhibits calcium oxalate growth. Patients with stones often have low citrate levels and excrete an abnormal form of GCI.14 Urinary inorganic pyrophosphate inhibits calcium phosphate crystal growth.12,16 Magnesium may inhibit calcium oxalate crystal growth because it forms a soluble complex with oxalate.
DISORDERS OF CALCIUM STONE FORMATION
BENIGN FAMILIAL “IDIOPATHIC” HYPERCALCIURIA
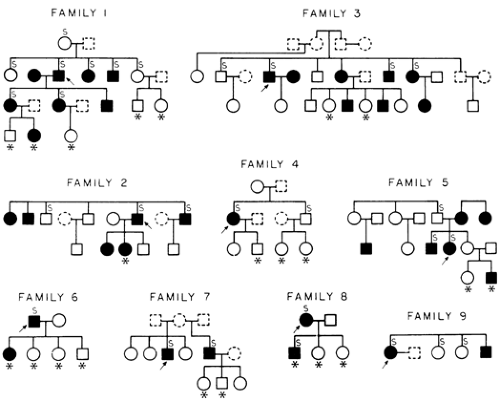
The abnormality that is found in 50% to 75% of patients with calcium stones is a normocalcemic hypercalciuria that is unexplained by any known systemic disease, such as sarcoidosis, hyperthy-roidism, rapidly progressive bone disease, vitamin D intoxication, immobilization, Paget disease, malignancy, or glucocorticoid excess.1,17 Called idiopathic hypercalciuria, it is more appropriately named benign familial hypercalciuria because it is found in the families of affected patients in a pattern that is compatible with an autosomal-dominant inheritance18,19,19a (Fig. 69-1). Familial hypercalciuria affects 2% to 4% of normal adults and accounts for ˜40% of stone formers. In ˜80% to 90% of patients, hypercalciuria is silent. The distribution of values for calcium excretion in the population is not bimodal, as expected for an inherited disorder, but non-Gaussian, with a long tail of high values. Therefore, the upper limit of normal for calcium excretion and the diagnosis of idiopathic hypercalciuria are arbitrary. An out-patient 24-hour urine collection is obtained; the patient eats ad libitum, including dairy products. Many investigators have determined calcium excretion under various dietary conditions that are important for studies of the pathogenesis of the disease. However, because changes in dietary calcium intake or the use of artificial diets change the urine chemistry, the authors believe that a free-choice diet is preferable when the diagnosis is in doubt. Hypercalciuria is determined by a 24-hour calcium excretion of above 300 mg for men and 250 mg for women, or either 4 mg/kg of body weight or 140 mg/g of urinary creatinine
in either sex. Hypercalciuria increases urine supersaturation with calcium oxalate or brushite.20,21
in either sex. Hypercalciuria increases urine supersaturation with calcium oxalate or brushite.20,21
The excessive urinary calcium excretion arises from increased intestinal calcium absorption.1 The pathogenesis of benign familial hypercalciuria is probably heterogeneous, with some patients overproducing 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the biologically active form of vitamin D that stimulates intestinal calcium absorption.22 Target organ hyper-responsiveness to 1,25(OH)2D3 may also be present, and elevated and normal serum 1,25(OH)2D3 levels have been reported.1 The apparent discrepancies of 1,25(OH)2D3 measurements among familial hypercalciuric patients may reflect their sensitivity to dietary calcium intake.23 The stimulus for increased 1,25(OH)2D3 production remains unknown, but low serum phosphorus or excess parathyroid hormone is unlikely. If dietary calcium is reduced severely, calcium balance becomes more negative than in normal people given an equivalent diet1; urine calcium excretion remains high in many of these patients24 (Fig. 69-2). This observation suggests that, under these conditions, calcium is being mobilized from bone. Because of this adverse response to a low calcium diet, which also occurs in normal people who are made hypercalciuric but not hypercalcemic by exogenous 1,25(OH)2D3, a low calcium diet is not advisable for these patients.25
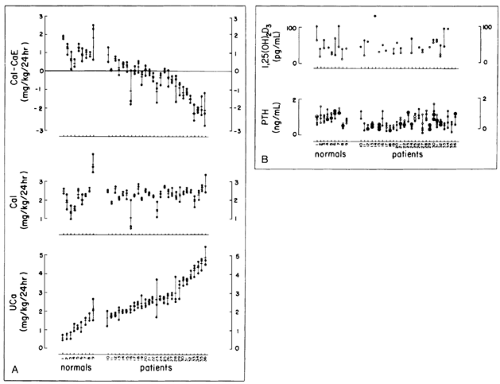 FIGURE 69-2. Calcium intake and excretion (CaE) (A) and serum parathyroid hormone (PTH) and 1,25(OH)2D3 (B) values in patients and normal subjects. Urine calcium excretion (UCa) and intake (CaI) are during low calcium diet. Serum values for 1,25(OH)2D3 and PTH during low calcium diet (Ca intake of 2 mg/kg body weight [BW] per day for 10 days, solid circles) and free-choice diet (open circles) are both shown. Mean calcium intake of normal subjects (2.29 ± 0.15 SEM in mg/kg BW of calcium in 24 hours) and patients (2.31 ± 0.05) did not differ. Mean excretion rates during low calcium diet (1.18 ± 0.11 vs. 2.87 ± 0.11, p <.001) for normal subjects and patients, respectively, and values of intake minus excretion (CaI – CaE, 1.14 ± 0.12 vs. -0.58 ± 0.11, p <.001) differed significantly from each other. Calcium excretion in normal subjects (1.78 ± 0.16) and patients (4.38 ± 0.15) during free-choice diet (not shown) differed from each other (p <.001) and from values during low calcium diet (p <.01, both normal subjects and patients). Normal subjects and patients are shown in order of ascending mean calcium excretion; numbers are for identification of individual normals and patients. [From Coe FL, Favus MJ, Crockett T, et al. Effects of low-calcium diet on urine calcium excretion, parathyroid function, and serum 1,25(OH)2D levels in patients with idiopathic hypercalciuria and normal patients. Am J Med 1982; 72:25.]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|