70.1
Introduction
Diet is an important modifiable risk factor for the prevention of osteoporosis. Studies have related several nutrients with bone health, such as vitamins A, B, C, D, E, K; minerals (potassium, magnesium, and silicon); and macronutrients (protein and fats) . Studies have also gone beyond single nutrient associations and linked foods, food groups, and dietary patterns with bone health. However, calcium and vitamin D are the primary nutrients considered for osteoporosis prevention and treatment in older adults.
Calcium is the most abundant mineral in the body contributing to critical functions related to vascular contraction, muscle function, nerve transmission, intracellular signaling, and hormonal secretion . Ninety-nine percent of the body’s calcium supply is stored in the bones and teeth where it supports structure and function. Bone acts as the calcium reservoir to maintain constant concentrations of calcium in the body. Vitamin D is a fat-soluble vitamin that is naturally occurring in very few foods. However, it is produced endogenously in the skin upon ultraviolet exposure. Biologically inert vitamin D, produced from sun exposure or obtained from diet or supplements, undergoes hydroxylation to convert to its active form, 1,25-hydroxy vitamin D or calcitriol. Vitamin D plays key roles in the body, including modulation of cell growth, neuromuscular and immune function, and the reduction of inflammation . It not only plays a role in bone growth and remodeling but also promotes calcium absorption in the gut and maintains adequate serum and phosphate concentrations for bone mineralization. With aging, there is a decline in calcium absorption and decline in serum 25-hydoxyvitamin D [25(OH)D] concentrations due to several reasons, which may in part increase the risk of age-related osteoporosis and consequent fractures in older adults. These adequate intakes of calcium and vitamin D are not only essential for osteoporosis prevention but also essential components of therapeutic treatments for osteoporosis. Although calcium and vitamin D have received significant attention, emerging evidence indicates that other nutrients also play an important role in skeletal health. The overview on nutrition and osteoporosis has been included in Chapter 21 , Nutrition and osteoporosis by Langsetmo et al., and nutrients beyond calcium and vitamin D are covered in Chapter 71 , Nutrients beyond calcium and vitamin D to treat osteoporosis by Nieves et al. The chapter discusses the interplay of calcium and vitamin D upon bone remodeling and reviews the data from human studies of calcium, vitamin D, and bone mineral density (BMD) and fractures. Finally, we also provide information on dietary recommendation of these two nutrients.
70.2
Bone remodeling, osteoporosis, and calcium
Calcium and vitamin D play key roles in bone health, which have been long supported by robust scientific evidence . Ninety-nine percent of the body’s calcium supply is stored in bone as mineral salt, a crystalline complex of calcium and phosphate called hydroxyapatite (Ca 10 (PO 4 ) 6 (OH) 2 ). Hydroxyapatite crystals comprise 70% of calcified bone and are responsible for its rigidity . The relative ratio of calcium to phosphorus content in these mineral crystals varies depending on nutritional status. Osteoblasts, the bone forming cells, stimulate the deposition of mineral salts into the bone. Hydroxyapatite crystals are deposited along, and close to, each segment of collagen fiber, thereby preventing the collagen fibers from slipping out of place. Hydroxyapatite strengthens the tough organic matrix composed of collagen fibers, which provides bone with flexible strength, while hydroxyapatite provides compressional strength. On the other hand, osteoclasts play a key role in bone resorption by releasing proteolytic enzymes and acids to dissolve bone and release calcium into the blood .
As detailed in the previous chapter by Heaney , the skeleton is a metabolically active organ that undergoes continuous remodeling. Bone remodeling involves the removal of mineralized bone by osteoclasts followed by the formation of bone matrix through the osteoblasts that subsequently become mineralized . There are three stages of the remodeling cycle: resorption, reversal, and formation . Osteoclasts are responsible for the resorptive stage, followed by reversal by mononuclear cells on the bone surface and bone formation by osteoblasts. The whole process can take up to 4 months. Bone homeostasis is maintained by a balance between the action of osteoblasts and osteoclasts . A range of growth factors regulate the activity of osteoblasts. Receptors for these factors are found on osteoblasts along with receptors for classic hormones, such as parathyroid hormone (PTH), parathyroid-hormone–related protein, and thyroid hormone. Additional nuclear steroid hormones are found on osteoblasts, including receptors for estrogens, androgens, vitamin D3, and retinoids . Osteoclast activity is mediated by binding of integrins, expressed in osteoclasts, with proteins on the surface of bone matrix. Their function is regulated both by locally acting cytokines and by systemic hormones .
Calcium homeostasis refers to the hormonal regulation of serum ionized calcium by PTH, 1,25-dihydroxyvitamin D (1α,25(OH) 2 ), and serum-ionized calcium itself, which together regulate calcium transport in the gut, kidney, and bone . Vitamin D and PTH act at three main sites to regulate extracellular calcium and phosphate: (1) small intestine, optimizing intestinal reabsorption of calcium; (2) kidneys, regulating renal excretion, and bone resorption independent of intestinal absorption; (3) bone, stimulating osteoblasts to produce a receptor called RANKL that ultimately stimulates osteoblasts to release calcium into the system ( Fig. 70.1 ) . Over time, increased PTH concentrations can lead to hypercalcemia and to significant bone loss, increasing the risk of fractures. PTH also stimulates resorption of phosphate from the bone but increases renal excretion of phosphate resulting in more phosphate excreted then resorbed. The net result of high PTH is that it decreases serum phosphate . Calcium absorption from dietary intake is dependent on several factors and only up to 30% of the calcium from food is actually absorbed . The efficiency of absorption is influenced by age, gender, vitamin D status and intake, other compounds present in food, and some medicines .
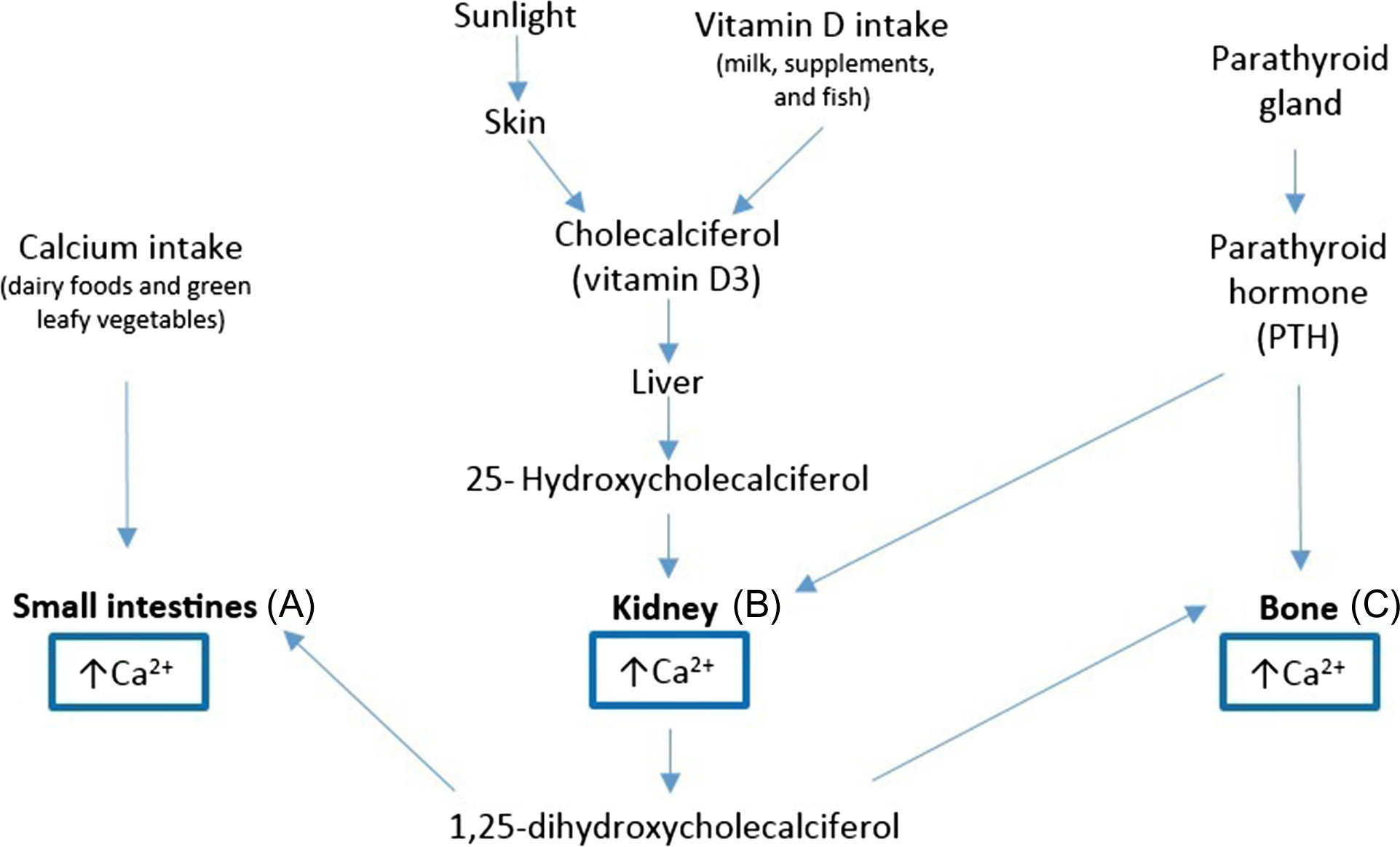
70.3
Interplay of calcium and vitamin D
Calcium intake, along with adequate vitamin D status, is essential to maintain serum calcium within normal physiological concentrations . Vitamin D active metabolite mediates calcium absorption from the ileum in the gastrointestinal tract and is also involved in the regulation of calcium resorption from bone. Vitamin D increases the formation of calcium-binding proteins in the intestinal epithelial cells, which transport calcium through facilitated diffusion .
Vitamin D is the general term for two different molecules, ergocalciferol (vitamin D 2 ) and cholecalciferol (vitamin D 3 ). Vitamin D is a unique nutrient in that it can be photochemically produced in the skin as well as ingested through diet. Sunlight is considered the main source of vitamin D as up to 90% of the total acquired vitamin D can come from endogenous production. Casual exposure of the skin to ultraviolet B radiation converts the vitamin D precursor in the epidermis (7-dehydrocholesterol) to previtamin D 3 , which quickly undergoes a thermal isomerization to become vitamin D 3 . With aging the ability to form vitamin D through sunlight decreases significantly, and supplements may become necessary to maintain adequate concentrations . The ability to form vitamin D through exposure of the skin to sunlight is also influenced by the amount of melanin in the epidermis, which is responsible for skin pigmentation and acts as a filter to UV rays. Therefore individuals with darker skin might require greater exposure to sunlight in order to produce enough vitamin D .
Vitamin D from all sources (intake and endogenously synthesized) is transported in the circulation to the liver, to be converted to 25-hydroxyvitamin D, or 25(OH)D, which has a half-life of 2–3 weeks and is considered the best marker of vitamin nutritional status ( Fig. 70.1 ), but can be also stored in the liver for several months. The final conversion of 25(OH)D into 1α,25(OH) 2 , its active form, occurs in the kidney’s proximal tubule. Vitamin D functions through a single vitamin D receptor. When serum calcium concentrations are low, calcium-sensing proteins found in the parathyroid gland stimulate the secretion of PTH, a potent bone-resorbing agent, in order to restore calcium normal concentrations . Only a very small amount (less than 0.03%) of the total vitamin D is free (in unbound form) .
70.4
Impact of calcium and vitamin D on bone mineral density
BMD is considered one of the main risk factors of fractures; hence, the correlation among calcium intake, vitamin D status, and BMD has been largely studied over the past decades . A considerable amount of human evidence supports the well-recognized and intuitive contribution of calcium for bone homeostasis maintenance and treatment strategies for osteoporosis. Human supplementation trials have also showed that higher calcium intake in the long-term could have relevant benefits to bone health and reduce the risk of osteoporosis by slowing the rate of bone loss associated with aging , and effects might be strongest in postmenopausal women, rather than perimenopausal (early stages) women . Observational studies as well as randomized controlled trials, as reviewed in the previous edition of this book , showed predominantly positive associations and effects of calcium and vitamin D supplementation on BMD and risk of fractures. In the following sections, we largely highlight studies published since the previous edition of this book.
70.4.1
Observational studies
70.4.1.1
Dietary calcium intake and bone mineral density: cross-sectional and longitudinal studies
A recent cross-sectional study analyzed data from the Korea National Health and Nutrition Examination Survey conducted in 2008–10 and included 7000 noninstitutionalized men and women (50 years of age or older). BMD of the lumbar spine (L1–L4) and femur, measured using dual X-ray absorptiometry (DXA) and calcium intake, was assessed using a 24-hour dietary recall questionnaire. The study reported that a calcium intake below 400 mg/day was associated with lower BMD and femoral cortical thickness. BMD in the lumbar spine and femoral neck was significantly lower with calcium intakes below 400 mg/day and intakes above 1200 mg/day were positively correlated with BMD ( Table 70.1 ) . The study data was obtained from a large nationwide survey and, therefore, can be considered representative; however, the cross-sectional nature of this study could not imply causality. Another cross-sectional study analyzed data from men and women from 19 different tightly controlled feeding studies that were conducted in a metabolic unit ( n =154 participants; women: 20–75 years; men: 19–64 years). This study estimated that about 741 mg of calcium consumed per day is needed to replace the calcium that is lost from the body metabolism ( Table 70.1 ) .
| Reference | Type of study | Population | n | Age (in years) | Exposure | Outcome measured | Results |
|---|---|---|---|---|---|---|---|
| Calcium | |||||||
| Kim et al. | Cross-sectional | Low calcium intake population (KNHANES) | 3448 men and 3812 women | >50 years | Ca intake | BMD | BMD in the lumbar spine (both sexes) and femoral neck (women) was lower when calcium intake was <400 mg/day |
| Hunt et al. | Cross-sectional | Men and women. Cross-sectional analysis of data from 19 controlled feeding studies | 154 | Women:20–75 years; Men: 19–64 years | Ca intake | Ca intake, fecal Ca and urinary Ca | Adults to consume about 741 mg/day of total calcium to replace the calcium that is lost from the body |
| Bristow et al. | Longitudinal (2 years) | Healthy men | 323 | >40 years | Ca intake | BMD | No association |
| Vitamin D | |||||||
| Bischoff-Ferrari et al. | Cross-sectional | Healthy men and women (NHANES III) | 13,432 | >20 years | Serum [25(OH)D] | BMD | Higher BMD associated with higher vitamin D status |
| Ensrud et al. | Prospective cohort (4 years) | Older men | 1279 | >65 years | Serum [25(OH)D] | Rate of change in BMD | Higher baseline serum 25(OH)D concentration was associated with reduced BMD loss |
In a longitudinal study, Bristow et al. determined the association of dietary calcium intake with BMD at the total body, hip, and spine and change in DXA-derived BMD over 2 years in 323 healthy men from New Zealand (mean age 57 years). Mean calcium intake as assessed using an abbreviated food frequency questionnaire (FFQ) was 870 mg/day [dietary reference intakes (DRI) of calcium for men aged 51–71 years: 1000 mg/day]. Dietary calcium was not related with BMD or bone loss over 2 years at any of the bone sites examined ( Table 70.1 ) . It is worth noting that this study cohort was from a randomized controlled trial of calcium supplementation on bone density. Inadequate sample size, inclusion of vitamin D sufficient men, and inadequate adjustment for specific confounders could have led to null finding in this study.
70.4.1.2
Vitamin D status and bone mineral density: cross-sectional studies
Serum 25(OH)D concentration has been largely associated with BMD. The latest report on DRI for Calcium and Vitamin D from the Institute of Medicine (IOM) concluded that an association between serum 25(OH)D concentrations and changes in BMD over time at the femoral neck can be supported by enough reasonable evidence from observational studies . In the large population-based National Health and Nutrition Examination Survey III (NHANES III), data were collected from 1988 to 1994 in 13,432 noninstitutionalized residents in the United States. In this study BMD (measured by DXA scan) was reported to be positively associated with serum 25(OH)D concentrations and increased with concentrations above 20 ng/mL, in younger and older adults, regardless of gender ( Table 70.1 ). Interestingly, in another analysis of the NHANES III data, which included 4958 community-dwelling women and 5003 men aged 20 years or older, in women with 25(OH)D concentrations below 20 ng/mL, higher calcium intake was associated with higher BMD at the hip. No association was observed in women with 25(OH)D concentrations above 20 ng/mL or in men. Overall among men and women, 25(OH)D status seemed to be the dominant risk factor of BMD relative to calcium intake .
70.4.1.3
Vitamin D status, bone mineral density, and fracture: prospective cohort studies
A 4-year multicenter prospective study with a focus on osteoporosis recruited older men (>65 years of age) from 2000 to 2002 in six regions of the United States. In a random sample comprising 1279 men, higher baseline 25(OH)D concentrations were associated with reduced DXA-derived BMD loss after an average of 4.4 years (hip and trochanter). Those with 25(OH)D concentrations below 20 ng/mL had larger subsequent rates of hip bone loss, but rates of loss were similar among men with higher serum vitamin D concentrations ( Table 70.1 ).
A prospective cohort study conducted in Melbourne (including 25% of participants born in southern Europe) investigated the long-term association between dietary calcium (assessed via food frequency questionnaire) and the incidence of self-reported fractures in 12,097 men and women aged 50 years or older over 13 years of follow-up. Participants in the highest quartile of dietary calcium (mean intake of 1348 mg/day) had 30% reduction in fracture risk compared to those in the lowest quartile of intake (mean intake of 473 mg/day, OR of highest vs lowest quartile: 0.70, 95% CI: 0.54–0.92) .
70.4.2
Randomized controlled trials of bone mineral density and fracture
Several randomized controlled trials have investigated the effect of calcium and vitamin D supplementation on BMD. Calcium and vitamin D supplements have been generally found to positively affect BMD in older adults. Some trials have assessed the effects of calcium or vitamin D alone as the intervention, and some have investigated calcium and vitamin D together as a combined intervention. It is important to consider these two types of intervention separately in order to better understand and determinate to what extent the effects should be attributed to each nutrient or their combination.
Of the three classic studies on this topic, two showed benefit of calcium and vitamin D supplementation on bone, while one showed small benefit for BMD but none for fractures. A randomized control trial by Dawson-Hughes et al. published in 1997 is considered one of the pivotal trials in demonstrating the efficacy of calcium supplementation in the prevention and treatment of osteoporosis. In this RCT of men and women over 65 years of age ( n =389), dietary supplementation with either 500 mg calcium plus 700 IU of vitamin D per day or placebo, moderately reduced bone loss measured in the femoral neck, spine, and total body over the 3-year study period. This study further showed a significant reduction in the first nonvertebral fracture in the supplemented group (500 mg calcium plus 700 IU vitamin D, n of fractures=26) compared to the placebo ( n of fractures=11) over 3 years of follow-up [RR: 0.4 (95% CI: 0.2–1.0; P =.03)]. However, these findings should be interpreted with caution given the small sample size.
Similarly, Chapuy et al. demonstrated a significant protective effect of 1200 mg of calcium and 800 IU of vitamin D daily supplementation over 18 months in reducing fracture risk in 3270 French elderly (mean age 82±6 years) living in nursing homes . The calcium plus vitamin D supplementation group also showed an increase in BMD at the proximal femur by 2.7%. This study showed a remarkable reduction (43%) in hip fractures in the calcium plus vitamin D supplementation group compared to the placebo in women over 18 months. A more recent confirmatory 2-year RCT on the effects of the same combined dose of calcium and vitamin D supplementation in 610 French older women reported a decrease of femoral neck BMD, in the placebo group only, after 12 months of treatment , clearly showing a benefit of these two nutrients upon bone density. A difference between intervention groups was also seen for hip BMD. However, it is important to note that in this study, the placebo group presented very low baseline serum 25(OH)D concentrations (mean 13±9 ng/mL) and low calcium intakes (~500 mg/day as assessed by frequency questionnaire). Consistent with the current debate of the influence of initial 25(OH)D concentrations, this may imply that efficacy of these nutrients observed might not be extendable to populations with less significant deficiency.
The Women’s Health Initiative (WHI) trial, with 36,282 healthy postmenopausal women (aged 50–79 years), reported a small but significant higher hip BMD in the calcium (1000 mg) plus vitamin D (400 IU) group compared to the placebo group over 7 years of follow-up, although no significant reduction in hip fracture, spine fracture, or total fractures was observed in this study . Some of this dilution of effect could be attributed to the lack of adherence and use of personal supplements by the study participants because sensitivity analysis to assess effect of nonadherence by censoring data from women who ceased to adhere to the study medication showed a significant reduction in hip fracture by 29% in the treatment group compared to the placebo group. This suggests that although calcium and vitamin D supplementation may be efficacious, it is likely to be more effective in older postmenopausal women over the age of 60 years at risk of fractures. However, authors also reported an increased risk of self-reported kidney stones in this study. It is important to note that in this population the average calcium intake was ~1150 mg/day and vitamin D serum concentrations around 46 nmol/L. The outcomes observed in this study may be due to the relatively high baseline vitamin D concentrations or the unsatisfactory compliance to treatment from participants reported by the study or variations on vitamin D baseline status. Subsequent analysis of WHI data further reported on fracture outcomes 4.9 years after the intervention stopped. Over an average of 11 years of follow-up, there was a 13% reduction in the risk of vertebral fractures among supplement users . Similarly, another study analyzed WHI data to examine long-term use of calcium and vitamin D supplements with an emphasis on fractures. Among women not taking personal calcium or vitamin D supplements at baseline, long-term calcium and vitamin D supplement use (≥5 years) was associated with 38% reduction in hip fracture risk compared with placebo group (RR: 0.62; 95% CI: 0.38–1.00). When this data was combined with WHI prospective observational study, there was a 35% reduction in hip fracture risk (RR: 0.65; 95% CI: 0.44–0.98) .
As outlined in Table 70.2 and discussed next, recent RCTs have further investigated the effect of both calcium and vitamin D 3 supplementation, either combined or not, in older populations.
| Reference | Population | Duration (months) | n | Age (years) | Vitamin D dose (IU/day) | Ca dose (mg/day) | Results |
|---|---|---|---|---|---|---|---|
| Calcium | |||||||
| Nakamura et al. | Healthy women | 24 | 450 | 50–75 | – | 500 or 250 mg of calcium or placebo |
|
| Rajatanavin et al. | Postmenopausal women | 24 | 404 | >60 | – | 500 mg or placebo |
|
| Vitamin D | |||||||
| Hansen et al. | Postmenopausal women | 12 | 221 | 60–75 | 800 IU daily or 50,000 IU twice monthly or placebo | – | No association |
| Reid et al. | Elderly ( ViDA study) | 24 | 452 | 50–84 | 100,000 IU monthly or placebo | – |
|
| MacDonald et al. | Postmenopausal women (The Aberdeen Study) | 12 | 305 | 60–70 | 400 IU; 1000 IU or placebo | – |
|
| Calcium plus vitamin D | |||||||
| Rahme et al. | Healthy elderly | 12 | 257 | >65 | 3750 or 600 IU | 1000 mg (both groups) | No significant differences in change BMD between the groups at any skeletal site. Compared to baseline, significant increments in percent change BMD at total hip and lumbar spine in both groups. No between groups change in BMD at any skeletal site. |
| Pop et al. | Postmenopausal women | 12 | 81 | 50–70 | 600, 2000, or 4000 IU | 1200 mg (all groups) |
|
| Smith et al. | Healthy women | 12 | 194 | 57–90 | 400, 800, 1600, 2400, 3200, 4000, or 4800 IU/day or placebo | To total calcium intake of ~1200 mg/day | No significant effect of daily vitamin D doses ranging from 400 to 4800 IU/day on BMD in older women who started with low serum 25(OH)D <50 nmol/L |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








