Breast: Locally Advanced (T3 and T4), Inflammatory, and Recurrent Tumors
Locally advanced breast cancer is defined by the 2009 American Joint Committee on Cancer staging criteria (see Chapter 24) as stage IIIA and IIIB disease.
Clinical or pathologic findings of locally advanced carcinoma at presentation include the following: tumor size greater than 5 cm; clinically or pathologically positive axillary lymph nodes; tumor of any size with direct extension to ribs, intercostal muscles, or skin; edema (including peau d’orange), ulceration of skin of breast, or satellite skin nodules confined to the same breast; inflammatory carcinoma (T4d); and metastases to ipsilateral internal mammary lymph nodes or ipsilateral axillary lymph nodes fixed to one another or other structures.
NATURAL HISTORY AND CLINICAL PRESENTATION
Locally Advanced (T3 and T4) Tumors
Locally advanced breast cancer often arises from a tumor that has been present for a long period of time thereby allowing growth within the breast and spread to nearby areas including lymph nodes, skin, and muscle. Infiltration of the deep lymphatics of dermis causes edema of the skin and increased breast size. Many patients will give a history of a breast or axial mass being present for years before they sought medical attention. More pronounced edema (peau d’orange) can indicate superficial and deep lymphatic involvement. Often, localized erythema is apparent throughout the breast. If left untreated this will result in ulceration and ultimately breast contraction, through tumor infiltration of Cooper’s ligament. Further extensive involvement includes satellite nodules and carcinoma en cuirasse, as if the patient were wearing a coat of armor in which the skin becomes plaque-like, thick and yellowish, red, or gray.
Less commonly, patients will report rapid onset and rapid enlargement of the breast with no specific palpable abnormality accompanied by redness and swelling. This is commonly referred to as inflammatory breast cancer. Often, biopsy of the skin covering the breast confirms tumor infiltration in the dermal lymphatics, or lymphatic tumor emboli, the hallmark of inflammatory breast cancer.
Metastatic spread can occur through lymphatic or hematogenous spread; the former involving the axillary, internal mammary or supraclavicular nodes and the latter causing spread to bone, lung, pleura, liver, and brain.
While their tumor characteristics may be different, inflammatory and locally advanced breast cancer are treated the same.
DIAGNOSTIC WORKUP
A thorough physical exam must be performed paying particular attention to appearance of the breast, fixation to the chest wall, evident lymphadenopathy and checking potential sites of spread. Radiographic evaluation of the breast will include mammogram and possibly ultrasound and magnetic resonance imaging (MRI). A repeat MRI may be particularly useful in evaluating response to neoadjuvant chemotherapy and to plan subsequent surgery.
Laboratory studies include a complete blood cell count, serum chemistry profile, including full liver function tests. Some clinicians find breast tumor markers such as CEA, CA 15-3 or CA 27-29 useful in monitoring response to treatment.
If liver function values are abnormal, a computed tomography (CT) scan of the abdomen should be obtained.
If anemia, leukopenia, or thrombocytopenia is present, bone marrow biopsy is necessary.
Radiographic studies include CT scan of chest abdomen and pelvis. Alternatively, some institutions utilize positron emission tomography (PET) (5) scans with or without CT to evaluate extent of disease.
Bone scans are generally used less frequently but may be recommended for stage III or IV disease to further delineate abnormalities on the above studies and should always be included if CT or PET is not performed.
If neurologic symptoms suggest cerebral metastases, a contrast-enhanced CT scan or gadolinium-enhanced MRI scan of the brain should be obtained; MRI is preferred if leptomeningeal spread of tumor is suspected.
PROGNOSTIC FACTORS
One of the most important prognostic factors is response to neoadjuvant treatment (3, 12, 20, 24, 28). Other factors associated with increased poor prognosis include larger, more diffuse tumors, presence of edema, and number of involved axillary nodes.
As with early stage disease, tumors that are estrogen and progesterone negative and have her-2 overexpression signify more aggressive disease.
GENERAL MANAGEMENT
Because of a compelling need for systemic therapy, multiagent chemotherapy plays a primary role in the treatment of these patients and therefore diagnosis is usually obtained by core biopsy and not surgical excision. Biopsy of any palpable lymph nodes should also be performed prior to initiation of chemotherapy to document malignancy.
Neoadjuvant chemotherapy (with or without hormone therapy) before surgical resection remains the cornerstone of treatment (29). Studies evaluating Adriamycin, Cytoxan chemotherapy pre- or postoperatively showed that there was no difference in overall survival; however, more patients were deemed resectable or were down staged to allow lesser surgery, for example, lumpectomy (6, 7, 24).
Subsequently, NSABP-27 has shown that the addition of a taxane (ACT) versus (AC) chemotherapy results in better complete pathologic response and disease free survival than AC alone. Further ACT → surgery improved response rates over AC → surgery → (1, 22). Here AC means preoperative deoxorubicin and cyclophosphamide.
Radiation therapy and surgery each have important roles in optimizing locoregional tumor control.
Surgery
Surgery should be performed on all patients with technically resectable disease after chemotherapy. Borderline resectable and unresectable locally advanced breast cancers should be treated with definitive radiation therapy to the breast and regional lymph nodes prior to surgery (27). In marginal cases, it is important to have the patient evaluated by both the surgeon and the radiation oncologist so a joint decision about the next phase of treatment can be formulated. If a course of initial radiation therapy is chosen, the patient should be evaluated at 5,000 cGy. If the tumor is resectable, then surgery should be performed.
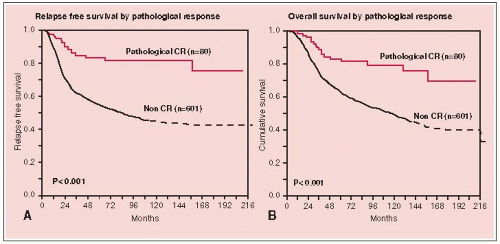
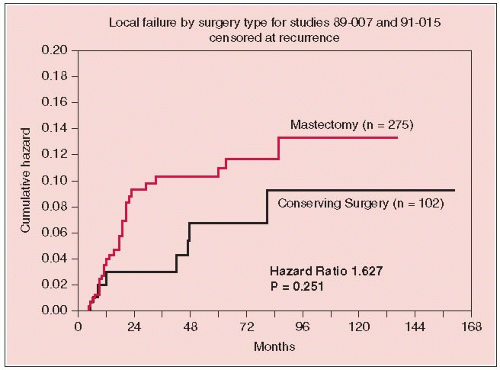
The treatment schema shown in Figure 25-1. Figures 25-2 and 25-3 illustrate relapse, overall survival, and local failure for patients treated with surgical resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree