Breast Imaging: Breast Cancer Screening, Diagnosis, Staging, and Surveillance | 1 |
Cristina Campassi, Lyn Ho, Jessica Galandak, Sergio Dromi, Divya Awal, Daniel Maver, Jasleen Chopra, and Sonya Y. Khan
INTRODUCTION
Imaging is essential in detecting, diagnosing, staging, and providing surveillance of diseases of the breast. Breast imaging plays a fundamental role, as clinical examination of the breast and surrounding regional lymph nodes is extremely challenging and nonpalpable abnormalities in the breast are common.
Indications for Breast Imaging
Asymptomatic women undergo screening while women with clinical breast signs and symptoms undergo a diagnostic evaluation.
SCREENING EXAM
Mammography is the main screening modality. Breast MRI is a supplemental modality in women at increased risk for breast cancer. Periodic screening is recommended. The National Comprehensive Cancer Network (NCCN) recommends receiving an annual mammogram starting at age 40 for average-risk women and earlier screening mammography supplemented with breast MRI for high-risk women (1,2). Screening exams are performed by a technologist and interpreted by a radiologist at a later time. The Food and Drug Administration requires mammography providers to inform women of their screening mammogram results within 30 days of the exam date. At our institution we batch-read screenings that are usually interpreted within a few days unless prior mammograms performed at another facility are unavailable for comparison. In such cases, 2 weeks are allowed to receive the patients’ prior mammograms before reading the current screening exam.
DIAGNOSTIC EXAM
Imaging evaluation is tailored to a specific clinical or imaging finding. The radiologist prescribes dedicated mammographic views and/or an ultrasound as needed and monitors the exam while it is in progress. The patient is informed of the results the same day.
Multimodality Breast Imaging
Breast imaging encompasses multiple modalities. Guidelines for the use of different modalities are outlined in the American College of Radiology (ACR) Appropriateness Criteria and Practice Guidelines (3–6).
MAMMOGRAPHY AND ULTRASOUND
Mammography and ultrasound are established conventional imaging modalities. In the screening setting, mammography is the only test proven to reduce mortality in randomized trials (7) and ultrasound has been shown to increase cancer detection in a subset of women with increased risk for breast cancer and dense breast tissue on mammography (8). In the setting of clinical symptoms, mammography and ultrasound are the first-line exam in women over and under the age of 30, respectively.
MRI
MRI is the best modality to detect silicone breast implant rupture. It is also the most sensitive imaging tool for detecting breast cancer and may identify cancer occult to clinical examination or conventional modalities such as mammography and ultrasound. MRI is used to supplement screening mammography in women with increased risk for breast cancer (2,9). In the setting of known breast cancer, MRI is used to assess the extent of disease in selected cases and response to systemic therapy in the neoadjuvant setting. In selected cases, it may be used for problem solving.
CONTRAST ENHANCED MAMMOGRAPHY (CEM)
Contrast enhanced mammography (CEM) is utilized in selected diagnostic cases, mostly as an alternative to breast MRI.
NUCLEAR MEDICINE MODALITIES
Nuclear medicine modalities such as positron emission mammography (PEM) and molecular breast imaging (MBI), also known as breast-specific gamma imaging (BSGI), are used for rare selected high-risk women in the diagnostic setting.
Breast Imaging Reporting and Data System (BI-RADS)
A standardized set of guidelines was developed by international experts in breast imaging with support from the ACR. The first edition, published in 1993, set guidelines for mammography. The most recent edition, published in 2013, provides guidelines for mammography, breast ultrasound, and breast MRI (10). The goal is to provide a common language for mammography and health care providers to facilitate communication and patient care. Standardization of finding description, assessment, recommendation, and reporting allows for easy communications, medical audit, and patient tracking.
BI-RADS PRINCIPLES
The guiding principle of BI-RADS is concordance. Finding descriptor, assessment, and recommendation need to be congruent (eg, a finding with a suspicious description cannot be classified as benign). Correlation of findings identified using different imaging modalities or at clinical breast examination (eg, a mass seen on mammogram correlated with ultrasound and clinical exam) is also required. Finally, desired benchmarks (eg, recall rate, sensitivity, specificity) are established and are reinforced through medical audit.
BI-RADS ASSESSMENT CATEGORIES AND RECOMMENDATION
At interpretation, findings are identified, analyzed, described, and then classified according to assessment categories. The assessment may be final or incomplete. A final assessment may apply to a screening or diagnostic exam. An incomplete assessment usually applies to a screening (eg, need comparison to prior mammograms or additional mammographic views). By convention, the assessment category is composed of a numeric code and a statement. Assessment category 0 is used for incomplete exams. The remaining six assessment categories, numbered from 1 to 6, are used for final assessment and span from negative exam to known malignancy (Table 1.1). The degree of abnormality and likelihood of malignancy are lowest with low BI-RADS assessment numeric code and highest for BI-RADS 5. The likelihood of malignancy for BI-RADS 3 is <2%, for BI-RADS 4 the likelihood ranges between >2% and 95%, and for BI-RADS 5 it is >95%. Notably, assessment category 4, used for suspicious findings, may be subdivided into three groups (ie, 4A, 4B, and 4C) based on level of suspicion (likelihood of malignancy is >2% to <10% for 4A, 10% to <50% for 4B, and >50% to 95% for 4C). The recommendation should be in keeping with the finding assessment category and clinical history. These guidelines ensure standardization across radiologists.
BREAST CANCER SCREENING
Breast cancer is the most common cancer in women of all races and ethnicities and a leading cause of premature mortality among U.S. women. Early detection is associated with reduced mortality. Mammogram is the primary test recommended to identify early breast cancer. Supplemental screening with other modalities, such as breast MRI and ultrasound, in selected subgroups of women has shown increased detection of breast cancer.
Table 1.1 BI-RADS Assessment Categories: Numeric Coding, Definition, and Pertinent Recommendation | |
Assessment category | Recommendation |
0. Incomplete | Need additional imaging evaluation (recall) and/or prior mammograms for comparison |
1. Negative | Routine mammogram |
2. Benign | Routine mammogram |
3. Probably benign | Initial short-term (6 months) follow-up |
4. Suspicious | Tissue diagnosis |
5. Highly suggestive of malignancy | Tissue diagnosis |
6. Known biopsy-proven malignancy | Appropriate action |
BI-RADS, breast imaging reporting and data system. | |
Mammography and Digital Breast Tomosynthesis
A meta-analysis of seven randomized controlled trials of screening mammography in women 39 to 74 years of age has demonstrated a 20% overall reduction in mortality from breast cancer with a 22% mortality reduction in women aged 50 to 74 and 15% reduction in women aged 39 to 49 (11). Despite the well-documented benefit of screening mammography, debate and uncertainty exist on the optimal screening strategy because exposure to screening generates false positives (ie, benign findings that require workup and biopsy to exclude cancer), identifies subclinical cancers that may not become clinically significant if undetected, and results in radiation exposure. As a result, recent screening strategies have deviated from the previously unanimous recommendation for annual screening mammography starting at age 40. The U.S. Preventive Services Task Force in 2009 and 2016 (12) and the American Cancer Society in late 2015 (13) have suggested more restrictive guidelines (Table 1.2). While recognizing the mortality reduction from screening mammography across all ages, both organizations encourage women to decide with their physician when to start and end, and how often to undergo screening mammography (12,13).
TECHNOLOGY
Mammography utilizes ionizing radiation, which is captured on a detector after passing through the breast. A major advancement in mammography technology over the past decade has been the development of digital technology, called full field digital mammography (FFDM) or 2D mammography. Compared to film screen, digital mammography uses a lower radiation dose, has a digital rather than film detector, and allows for separation of image acquisition, display, and archiving. The most recent improvement in mammography is digital breast tomosynthesis (DBT). While 2D mammography administers ionizing radiation through a stationary source perpendicular to the breast, DBT uses a moving x-ray source to image the breast at different angles. Thus, DBT obtains multiple digital images that can be reconstructed to obtain a quasi-tridimensional (3D) representation of the breast as opposed to FFDM, which obtains a bidimensional (2D) image. As a result, while breast tissue is superimposed on a view obtained with FFDM, it is viewed separately with DBT. This improves lesion detection and characterization while decreasing superimposition of breast tissue and the rate of false positives.
TECHNIQUE
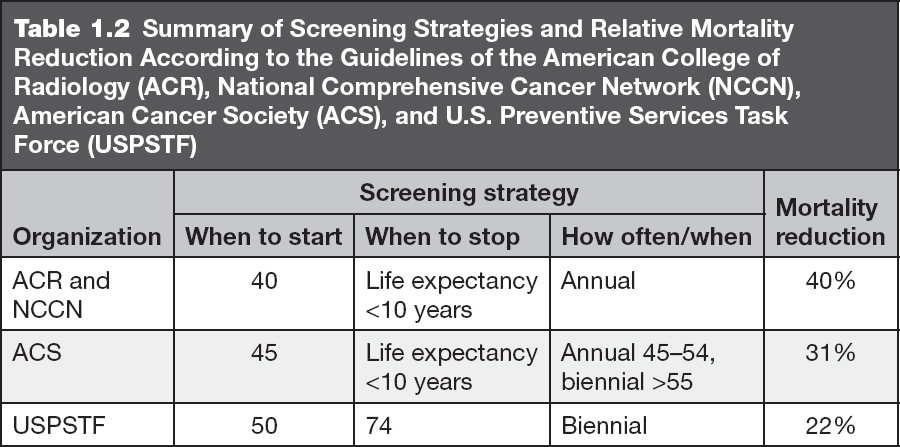
The same standard technique is used regardless of the technology utilized, film-screen mammography, FFDM, or DBT. A mammogram is performed with the patient standing or, if needed, sitting in a chair. The mammographic views are acquired with the breast under compression. A screening mammogram includes projections of each breast in two routine views: craniocaudal and mediolateral oblique (Figure 1.1). Therefore, typically a bilateral screening mammogram includes four views.
INTERPRETATION
The radiologist analyzes the mammographic views and classifies a screening mammogram as negative, benign, or incomplete, using the BI-RADS assessment categories 1, 2, and 0, respectively. Approximately 10% of screening mammograms are incomplete and require additional imaging with dedicated mammographic views and/or ultrasound in accordance with the ACR Practice Guidelines (4,5). Mammographic interpretation and detection of breast findings depend on breast composition (Figure 1.2). As the relative amount of fat decreases and glandular tissue increases, the breast becomes dense on a mammogram and cancer detection becomes more challenging (Figure 1.3). Additionally, dense breast tissue is considered an independent risk factor for breast cancer. Legislations to increase women’s awareness of breast density and its effect on screening mammography in the setting of dense breast tissue has been passed in the majority of U.S. states since 2009. This state legislation requires mammography providers to share information about breast density and/or inform women of their own breast density. Federal legislation may follow as the Breast Density and Mammography Reporting Act introduced to the U.S. Congress in October 2013 and to the U.S. Senate in February 2015 is under consideration.
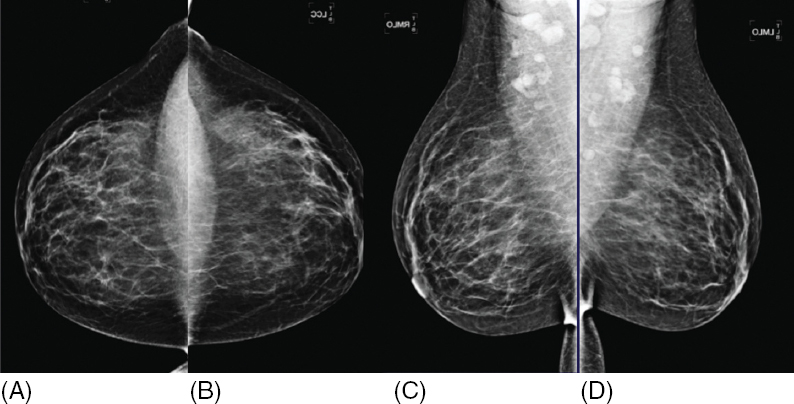
Figure 1.1 Digital mammogram with 2D technique. Screening mammogram includes two views of each breast: craniocaudal (CC) (A, B) and mediolateral oblique (MLO) (C, D). For viewing and interpretation, the mammographic views are displayed side by side to facilitate comparison of breast tissue and identification of findings that may represent breast cancer.
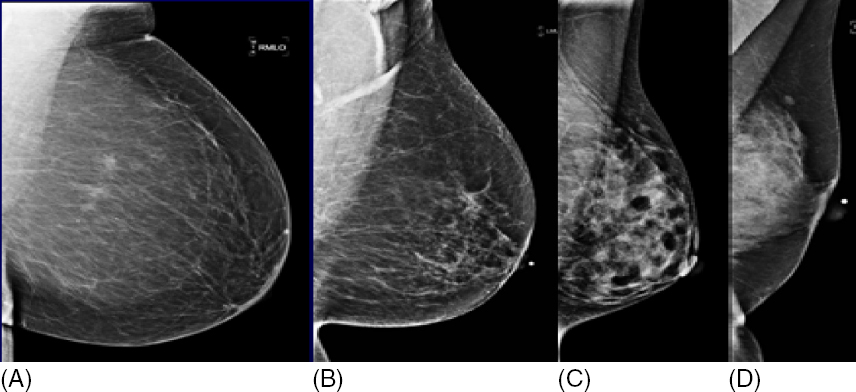
Figure 1.2 Breast composition varies depending on the amount of fatty and glandular tissue. Classification of breast composition ranges from almost entirely fatty (A) to extremely dense (D). The majority of women have either scattered fibroglandular elements (B) or heterogeneously dense breast tissue (C) while less than 20% of the female population has either fatty (A) or extremely dense breast tissue (D).
FULL FIELD DIGITAL MAMMOGRAPHY (FFDM) AND DIGITAL BREAST TOMOSYNTHESIS (DBT)
Compared to film screen, FFDM has shown improved cancer detection of 15% in women under the age of 50 years, women with radiographically dense breast tissue, and premenopausal or perimenopausal women (14). DBT has further increased breast cancer detection by 27% to 40% and decreased false-positive rates by 15% to 40% compared to FFDM (15).
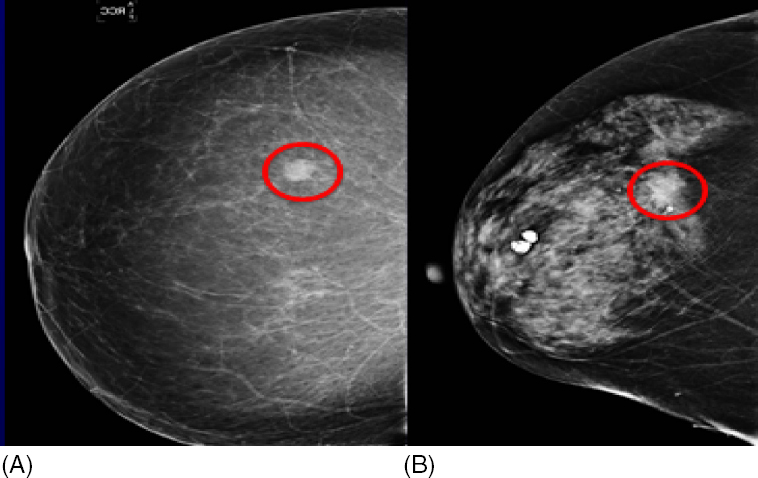
Figure 1.3 Breast composition influences breast cancer detection. A breast cancer (circle) is easier to detect in a fatty breast (A) compared to a dense breast (B).
Breast MRI
Several trials performed throughout the United States, Canada, and Europe in the mid- to late 1990s assessed the benefit of adding screening MRI to annual mammography in women who were at increased risk of breast cancer. The consistent finding was a much higher sensitivity for MRI when compared to mammography with sensitivities between 71% and 100% with MRI and 16% and 40% with mammography. The specificity of mammography remained higher than MRI, ranging from 93% to 99% compared to 81% to 99% for MRI. While the combination of higher sensitivity and lower specificity with MRI results in higher callback and biopsy rates than mammography, it also results in a higher cancer detection rate (1.04% vs. 0.46% in the Netherlands trial and 1.44% vs. 0.69% in the UK trial).
TECHNOLOGY
MRI utilizes magnetic fields to create multiplanar cross-sectional images through the body. MRI does not utilize radiation and has extremely good soft tissue contrast resolution, making it an excellent imaging modality for evaluating the breast. An intravenous gadolinium-based contrast agent is needed to reliably detect cancers, cancer extension, and other lesions.
TECHNIQUE
Patients are positioned prone. Both breasts are accommodated within an open padded platform (coil) that allows imaging without compression. The patient is placed into the bore of the MRI machine. Claustrophobic patients may need open magnets or premedication with anxiolytic. Multiple sequences are performed including precontrast and postcontrast. The overall scan time is usually 15 to 25 minutes. Although the scan requires that the patient be able to hold still during the entire examination, sedation is not required. Contraindications to MRI include a prior allergic reaction to contrast or severe renal insufficiency. The use of gadolinium is not recommended if the glomerular filtration rate (GFR) is below 30 mL/min due to the risk of nephrogenic systemic fibrosis (16). The use of gadolinium-based agents for elective exams such as breast MRI is contraindicated during pregnancy and is not needed if the examination is performed to evaluate for silicone breast implant rupture only.
RECOMMENDATIONS
The American Cancer Society guidelines for screening breast MRI as an adjunct to mammography (9), the NCCN (2), and the practice guidelines of the ACR (6) are used as a reference across specialties in the medical community. In our practice, screening breast MRI is used for all groups of high-risk women listed in the American Cancer Society guidelines (see Table 1.3). In particular, our experience on breast cancer survivors has shown that when breast MRI is used in adjunct to mammography, cancer detection rate increases compared to mammography alone; see Figure 1.4.
Breast Ultrasound
Historically ultrasound has been used as a diagnostic problem-solving tool in patients who present with either a physical finding (nipple discharge, palpable lump), a mammographic finding (asymmetry, mass), or, less commonly, an MRI finding (second look ultrasound for enhancing mass) (see “Imaging Assessment After Breast Cancer Diagnosis”). It is also the main modality for image-guided procedures (see “Image-Guided Breast Biopsies”). In addition, breast ultrasound has been investigated as a modality for breast cancer screening (see this section).
Table 1.3 American Cancer Society Guidelines for Supplemental Screening Breast MRI |
Evidence-based recommendation for annual screening MRI BRCA mutation carrier First degree relative of BRCA carrier, but untested Lifetime risk for breast cancer >20% when evaluated with a model dependent on family history |
Consensus expert opinion recommendation for annual screening MRI Radiation to the chest between age 10–30 years Patients with Li–Fraumeni, Cowden, or Bannayan–Riley–Ruvalcaba syndromes and their first degree relatives |
Insufficient evidence for or against annual screening MRI Lifetime risk for breast cancer of 15%–20% when evaluated with a model dependent on family history Lobular carcinoma in situ or atypical lobular hyperplasia Atypical ductal hyperplasia Heterogeneously or extremely dense breast on mammography Women with a personal history of breast cancer, including ductal carcinoma in situ |
Consensus expert opinion recommendation against annual MRI screening Lifetime risk for breast cancer <15% |
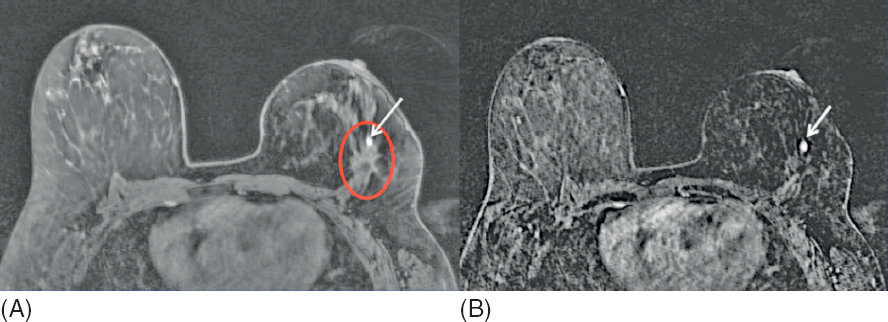
Figure 1.4 MRI detected, mammographically occult, local recurrence in a high-risk woman with personal history of breast cancer treated 5 years earlier with partial mastectomy and radiation therapy. (A) Postcontrast MR image shows a 5-mm circumscribed homogeneously enhancing round mass (arrow) along the anterior aspect of the operative bed (circle). Asymmetric size, contour, and skin thickening of the left breast are due to prior treatment for breast cancer. (B) Subtracted postcontrast MR image better shows the focal nature of the enhancing mass (arrow). MR-guided breast biopsy shows ductal carcinoma in situ (DCIS).
TECHNOLOGY
Ultrasound is an imaging modality that uses sound waves to create cross-sectional images through the body. Ultrasound does not use radiation, is portable and inexpensive, and produces excellent imaging of the breast.
a. Grayscale ultrasound shows the tissue in different shades of gray and is best for anatomic evaluation and characterization of lesion morphology (Figure 1.5).
b. Color Doppler ultrasound is best for evaluating the presence of blood flow and distinguishing venous and arterial flow.
c. Power Doppler ultrasound is best for detecting a subtle amount of blood flow without the capability of differentiating between arterial and venous flow.
d. Ultrasound elastography allows quantitative and qualitative evaluation of tissue deformation in response to an applied force. This is a simple and rapid method that can improve the sensitivity and specificity of grayscale images.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


