The condition should be a significant health problem
The natural history should be understood
There should be an early or latent stage
Treatment at an early stage should be of more benefit than started at a later stage
There should be a suitable test
Test should be acceptable to the population
Screening should be repeated at intervals
Facilities available for diagnosis and treatment
Chance of harm should be less than chance of benefit
Cost effective
13.2 Principles of Screening
13.2.1 The Condition Being Screened for Should Be an Important Health Problem
13.2.2 The Natural History of the Disease Should Be Well Understood
The natural history of breast cancer is not completely understood. Many appear to originate at a preinvasive stage, ductal carcinoma in situ (DCIS), which may subsequently progress to invasive breast cancer. Evidence for this includes:
A significant proportion of invasive breast cancers having associated DCIS
Autopsy studies estimating that approximately one-third of DCIS progresses to invasive cancer [4]
Studies where DCIS was initially misdiagnosed as a benign lesion and later recognised to be DCIS and where invasive cancer subsequently developed in a significant proportion of women (15–75%) [5, 6]
However, it is unclear why some, but not all, DCIS progresses to invasive cancer and why some invasive breast cancers arise de novo with no DCIS.
13.2.3 There Should Be a Detectable Early Stage
Cancer screening aims to detect cancer before symptoms appear. Screening is not useful for all cancers, and for the disease to be amenable to screening, there must be a latent period during which it would be possible to detect the disease before it reaches an advanced stage.
Breast cancer is a heterogeneous disease both with regard to variation among tumours and also the cellular composition within individual tumours. Two events are thought to occur in its natural history and progression: early dissemination and phenotypic progression [10]. If one or both events are related to the size of the breast tumour, then early detection of the tumour may prevent dissemination of tumour cells or the development of a more aggressive tumour that may lead to improved prognosis.
For breast cancer, there is a detectable preclinical phase where tumours are visible on mammograms before they become palpable. Tumours detected are more likely to be non-invasive, and if already invasive less likely to have regional or distant spread.
13.2.4 Treatment at an Early Stage Should Be of More Benefit than at a Later Stage
Screening aims to identify asymptomatic individuals at an early point in their natural history when delivery of early treatment could potentially reduce mortality. Direct comparison of outcomes in women presenting with symptomatic breast cancer is not valid without taking into account three potential biases:
- 1.
Lead time bias – the apparent improvement in survival that is seen when screening advances the time of diagnosis to time of death without any change in the actual time of death, merely reflecting a greater period of time that the individual is aware of the presence of cancer due to earlier detection.
- 2.
Length-time bias – screening has the potential to pick up more cancers that are less aggressive and slow growing as opposed to rapidly growing aggressive cancers which are more likely to present symptomatically in-between screens.
- 3.
Selection bias – women who attend for screening are more likely to be heath aware than those who decline and will probably have a better prognosis regardless of the offer of screening.
These biases can potentially be removed in randomised trials of population-based screening as described later.
13.2.5 A Suitable Test Should Be Devised for the Early Stage
A screening test should detect the majority of women who have cancer (high sensitivity) while eliminating most women who do not have cancer (high specificity). Sensitivity is defined as the proportion of all women with breast cancer who test positive. Specificity is defined as the proportion of all those without breast cancer that test negative. An ideal screening test should have 100% sensitivity and specificity, but in reality no screening test will achieve this. Different modalities for breast cancer screening are described later, but mammography has been the commonly used modality for general population-based screening programmes due to its combined high sensitivity and specificity compared to other screening modalities.
13.2.6 The Test Should Be Acceptable
The success of any screening programme will be dependent on the acceptability of the screening test to the population being screened. This will be reflected by the rate of uptake following an invitation to attend for screening. In an overview of mammographic screening programmes operating in Europe for the period 2005–2007 [11], 13 of the 26 programmes achieved the European Union benchmark of acceptable participation (>70%), and 9 achieved the desirable level (>75%) [12]. In England in 2014–2015, 2.11 million women aged 45 and over were screened by the NHS Breast Screening Programme, with an overall uptake of 71.3% [13].
13.2.7 Intervals for Repeating the Test Should Be Determined
In order to be effective, screening should be repeated at regular intervals. This is a balance between the cost and practicality of screening too frequently and minimising the number of interval cancers that present between screens.
There is variation with regard to the time interval between mammographic screens across EU states, most adopting a policy of biennial mammograms [14], while in the UK women are invited every 3 years.
Growth time or lead time is an important factor with regard to deciding on frequency of screening [15]. Younger women are more likely to have cancers with a shorter growth time potentially increasing interval cancers as opposed to an increased likelihood of slower growing cancers in older women. So theoretically younger women should have a shorter interval between screens compared to their older counterparts. The UK breast cancer frequency trial showed no benefit for women aged 50–62 years of annual mammograms over 3 yearly mammograms [16]. The study did show that tumours diagnosed in women in the study arm were significantly smaller, but there was no significant difference in terms of nodal status, histological grade and predicted mortality between the two groups.
13.2.8 Adequate Health Service Provision Should Be Made for the Extra Clinical Workload Resulting from Screening
The impact of the introduction of breast screening programmes across Europe has been the development of breast multidisciplinary teams both for the diagnosis and treatment of breast cancer. The use of triple assessment for diagnosis in breast care originated in breast screening practice and has subsequently been widely adopted in symptomatic practice.
13.2.9 The Risks, Both Physical and Psychological, Should Be Less than the Benefits
Potential physical and psychological hazards of screening using mammography include:
- 1.
Radiation exposure – use of x-rays for mammography exposes women to low doses of ionising radiation that could cause breast cancers. The actual dose of radiation depends on several factors including the number of views of each breast and whether film or digital mammography is used.
It has been estimated that screening women every 3 years from age 47–73 in the UK breast screening programme may cause 3–6 cancers per 10,000 women screened [17]. Digital mammography, which uses a lower radiation dose, is now more commonly used in screening programmes, and it is likely that this risk is now reduced.
- 2.
Overtreatment – unnecessary treatment following the diagnosis of tumours that would never have been of clinical significance in a woman’s lifetime. This is discussed in more detail later.
- 3.
Pain – the breast is compressed and flattened when carrying out mammography in order to improve the image quality and reduce the radiation dose. A substantial proportion of women find this painful, and there is evidence that painful mammography contributes to non-re-attendance for breast screening [18].
- 4.
False-positive results – a proportion of women are recalled following mammography and found not to have cancer. This is called a false-positive result. Recall rates vary between different screening programmes and will be higher at the first prevalent screen than subsequent incident round screens. In England in 2014–2015, for women aged 45 and over screened by the NHS Breast Screening Programme, 7.8% were recalled at their prevalent screen and 3.0% following an incident round screen [13].
Of the women recalled and found not to have cancer, the majority will only have further imaging (ultrasound, further mammography). Some will have a breast biopsy, usually a core biopsy under local anaesthetic but occasionally a formal open surgical biopsy under general anaesthetic. Numerous studies have assessed the psychological impact of a false-positive result on women with conflicting results. A recent systematic review [19] concluded that, in the population at general risk of breast cancer, a false-positive result can cause breast cancer-specific psychological distress, lasting for up to 3 years.
- 5.
False-negative results – no screening test is completely accurate, and sometimes mammographic screening will not detect a breast cancer. This can give a false sense of reassurance and potentially lead to delays in treatment. This may occur because the cancer is mammographically occult or develops as an interval cancer between screening rounds. Women should be warned of this possibility in literature given prior to breast screening.
13.2.10 The Costs Should Be Balanced Against the Benefits
It is important to continually compare the cost-effectiveness of health screening programmes against other healthcare provisions. This can be expressed as cost per quality-adjusted life year (QALY) gained. This has proved difficult to accurately achieve for breast screening due to ongoing uncertainty regarding the overall benefits and the development of breast screening over time (e.g. one- to two-view mammography, age extension, film to digital conversion, etc.). An assessment of the cost-effectiveness of the UK NHS Breast Screening Programme concluded that there was a moderate probability of breast screening being cost-effective at a willingness-to-pay threshold of £20,000 per QALY [20] but that further research was required.
In summary, breast cancer is a significant health problem, the natural history of which is sufficiently understood for the application of mammographic screening with the aim of early detection and treatment of cancer so as to improve outcome. Mammography as a population-based screening modality is well accepted in Europe and the UK as evidenced by the relatively high participation rate as previously depicted. Mammography is a reliable test (high sensitivity and specificity) in the group undertaking screening, and the subjects are relatively unharmed with the minimal radiation exposure especially with the widespread adoption of digital mammography across Europe.
13.3 Breast Cancer Screening Modalities
Three main screening methods have been assessed in population-based breast screening trials:
- 1.
- 2.
- 3.
Table 13.2
Randomised trials of breast cancer screening
Start date | Number of women | Age group (years) | Invited group | Control group | Attendance | ||
|---|---|---|---|---|---|---|---|
Method | Interval (months) | ||||||
New York HIP | 1963 | 62,000 | 40–64 | M | 12 | nil | 65% |
CE | |||||||
Malmo I and II | 1976 | 60,076 | 45–69 | M | 18–24 | nil | 74% |
Swedish Two County | 1977 | 133,065 | 40–74 | M | 24–33 | nil | 85% |
SE | |||||||
Canada I and II | 1980 | 89,835 | 40–49 and 50–59 | M | 12 | CE and SE | 88% |
CE and SE | |||||||
Stockholm | 1981 | 60,800 | 40–64 | M | 24–28 | nil | 82% |
Gothenburg | 1982 | 52,222 | 40–59 | M | 18 | nil | 84% |
UK Age Trial | 1991 | 160,921 | 39–41 | M | 12 | nil | 81% |
Edinburgh | 1978 | 54,654 | 45–64 | M | 24 | nil | 65% |
CE | |||||||
A study of one- versus two-view mammography has shown that the addition of a second craniocaudal view increases both the sensitivity of screening during the prevalent round as well as reducing recall rates [27].
More recently there has been a shift to full-field digital mammography (FFDM) rather than analogue (film) mammography for breast screening. The prospective OSLO II Study demonstrated that FFDM resulted in a significantly higher cancer detection rate than film mammography in a population-based screening programme [28]. All services in the UK NHS Breast Screening Programme now use digital mammography, and this is also the case in a large number of diagnostic centres across Europe. Images are shown on high-resolution monitors and are able to be digitally transferred and stored providing more efficient screening services.
A newer development in breast cancer screening is the use of digital breast tomosynthesis (DBT) which is a three-dimensional mammography technique. The STORM Study demonstrated that the addition of DBT to mammography increases rates of detection of both in situ and invasive cancers and may reduce recall rates [29]. DBT is being increasingly used for breast screening assessment (see ◘ Fig. 13.1). No reduction in breast cancer mortality has been demonstrated to date using DBT, but further trials are planned.
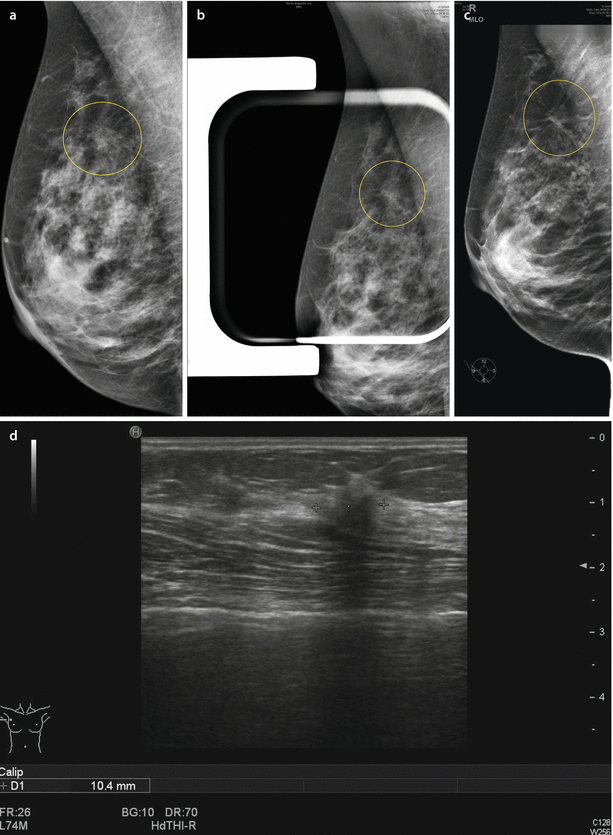

Fig. 13.1
Use of digital breast tomosynthesis in breast screening assessment. a Screening mammogram (oblique view) showing possible parenchymal deformity. b Mammogram compression view where deformity is subjectively less obvious (falsely reassuring). c Digital tomosynthesis slice image clearly showing the deformity giving diagnostic confidence. d Ultrasound scan confirming abnormality (invasive lobular carcinoma on biopsy) (Courtesy of Dr. M Bagnall)
Ultrasound has been shown to be a useful adjunct to mammography in increasing sensitivity and detection rate of early cancers in younger women [30].
13.4 Evidence from Breast Cancer Screening Trials
Evidence from randomised controlled trials (RCTs) is important in assessing the efficacy of breast screening in terms of mortality reduction from breast cancer.
The Health Insurance Plan study in 1963 was the first breast cancer screening trial randomising women aged 40–64 years that were enrolees in the Health Insurance Plan of Greater New York to either study (annual mammography screening and clinical breast examination for four consecutive years) or control groups [32]. At 18 years after entry, the study group had about a 25% lower breast cancer mortality among women aged 40–49 and 50–59 at time of entry than the control group. However, to a large extent, the difference among the 40–49-year-olds occurred in the subgroup diagnosed with breast cancer after age 50 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree