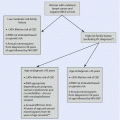
Fig. 42.1
Algorithm for the management of women with breast cancer during pregnancy
42.2 Diagnosis and Staging
BCP generally presents at a more advanced stage at diagnosis as compared to breast cancer in the general population [26]. The possible delay in diagnosis is related to the fact that pregnancy increases breast density, making clinical examinations and mammography more difficult to evaluate [27–29]. Physicians should be aware about the possibility that a breast lump in a pregnant patient may be associated with a cancer diagnosis: in these cases, imaging and pathological examination should be performed without delay [25]. Histopathological diagnosis based on core biopsy represents the gold standard for BCP and should follow standard procedures as in non-pregnant patients, but the pathologist needs be informed about the pregnancy status [11, 25] (◘ Table 42.1).
Table 42.1
Recommendations for the treatment of women with breast cancer during pregnancy
Intervention | Type of intervention | Recommendation |
|---|---|---|
Diagnosis and staging | Core biopsy | Recommended; the pathologist needs to be informed about the pregnancy |
Mammography with abdominal shielding | Recommended | |
Breast ultrasound | Recommended | |
Contrast-enhanced MRI | Not recommended | |
Chest X-ray with abdominal shielding | Recommended, if indicated | |
Ultrasound for abdomen and pelvis | Recommended, if indicated | |
MRI without gadolinium | Possible, if indicated | |
Computed tomography, bone scan and positron emission tomography | Not recommended | |
Local treatment | Breast-conserving surgery or mastectomy | Recommended |
Sentinel lymph node biopsy | Possible | |
Immediate breast reconstruction | Tissue expander insertion seems feasible, but limited data are available | |
Radiotherapy | Not recommended | |
Systemic treatment | Chemotherapy | Recommended in the second and third trimesters (not to be administered after week 34 of gestation). Anthracyclines plus cyclophosphamide is the preferred regimen. Taxanes can be administered (paclitaxel to be preferred). 5-Fluorouracil and dose-dense regimens are not indicated |
Anti-HER2 agents | Not recommended | |
Endocrine therapy | Not recommended | |
5-HT3 antagonist | Recommended; ondansetron to be preferred | |
Corticosteroids | Not recommended in the first trimester, can be used in the second and third trimesters (methylprednisolone to be preferred) | |
NK1 antagonist | Not recommended | |
G-CSF | Not recommended |
Imaging procedures for diagnosis and staging should aim to limit exposure to ionizing radiation, and the benefits of each modality versus the potential risks to the foetus should always be taken into account [11, 25]. Breast ultrasound as well as mammography with abdominal shielding can be safely and effectively performed in pregnant patients [30, 31], while insufficient data are available about the diagnostic accuracy and safety of contrast-enhanced breast magnetic resonance imaging (MRI) which is not recommended for BCP [11, 25] (◘ Table 42.1). Ultrasound represents the preferred imaging modality for staging the abdomen and pelvis, and chest X-ray with abdominal shielding can be performed to stage the chest [25] (◘ Table 42.1). In the cases of suspected bone or brain metastases or if the other imaging procedures were inconclusive, MRI without gadolinium can be considered [25] (◘ Table 42.1). Computed tomography, bone scan and positron emission tomography should be avoided during pregnancy [11, 25] (◘ Table 42.1).
As recommended by the Panel of the Second International Consensus Conference on Breast Cancer in Young Women (BCY2), genetic counselling should be offered for every young breast cancer patient, especially if there is a family history of breast carcinoma or a diagnosis of TNBC [32]. The probability of detecting BRCA mutations in patients with TNBC is approximately 20% [33]. Since women with BCP are diagnosed at a young age and the majority of them with a TNBC, genetic counselling may be indicated in many of these patients. Due to the direct influence on breast cancer management in terms of both local and systemic treatment, rates of BRCA mutation testing are increasing in young breast cancer patients and will probably become even more common in the coming years [34].
42.3 Local Treatment
42.3.1 Surgical Management
Surgery can be safely performed at any time during the course of gestation, and the surgical approach (i.e. radical vs. conservative surgery) should follow the same guidelines as for non-pregnant cases [11, 25] (◘ Tables 42.1 and 42.2). Mastectomy is not mandatory for patients with BCP solely on the basis of possible delay in the delivery of radiotherapy [11, 25]. Although the available published data on breast conservation are limited, they support the safety and feasibility of this procedure in pregnant patients [35]. However, patients diagnosed in the first trimester who desire to conserve the breast should be informed that a possible increased risk of local recurrence due to the long delay in postoperative radiotherapy cannot be ruled out [35].
Table 42.2
Treatment options during the different trimesters of pregnancy
First trimester | Second trimester | Third trimester | After delivery | |
|---|---|---|---|---|
Breast surgery | Yes | Yes | Yes | Yes |
Sentinel lymph node biopsy | Yes | Yes | Yes | Yes |
Radiotherapy | No | No | No | Yes |
Chemotherapy | No | Yes | Yes (no after week 34) | Yes |
Anti-HER2 therapy | No | No | No | Yes |
Endocrine therapy | No | No | No | Yes |
According to the American Society of Clinical Oncology (ASCO), clinicians should not recommend sentinel lymph node biopsy (SLNB) in patients with BCP [36]. However, the use of lymphoscintigraphy with technetium-99 SLNB has been shown to be safe and feasible during pregnancy [37–40] (◘ Tables 42.1 and 42.2). The 1-day protocol is associated with a negligible dose to the foetus (i.e. 0.014 mGy or less), much lower than the limit established by the United States (US) National Council on Radiation Protection and Measurements [41]. Hence, specific guidelines for patients with BCP suggest that SLNB rather than axillary clearance should be offered whenever indicated [11, 25]. Blue dye for mapping should be discouraged in pregnant patients due to the low but potentially harmful risk of anaphylactic reaction [11, 25].
For breast cancer patients who undergo mastectomy, immediate breast reconstruction as compared to no reconstruction offers the same survival rates and should be offered to all patients with the exception of those with inflammatory breast cancer [32]. In women with BCP, only one series of 13 cases showed the feasibility of tissue expander insertion with a short operation time and no significant morbidity to both the patient and the foetus [42]. This surgical technique could be considered in this scenario [11], but patients should be aware about the limited data on this topic (◘ Table 42.1). Lengthy autologous flap based procedures should probably be avoided.
42.3.2 Radiotherapy
The exposure of the foetus to radiation therapy can cause several adverse effects (i.e. intrauterine growth restriction, mental retardation, risk of childhood cancer, foetal death) [25].
Although some successful cases of radiotherapy for BCP with the subsequent birth of healthy children have been reported, the available data are too limited to draw solid conclusions [35]. Despite the fact that the radiation doses needed for adjuvant breast irradiation should be lower than the threshold for causing foetal toxic effects, it is preferable to postpone its use until the postpartum period [25] (◘ Tables 42.1 and 42.2).
Foetal dosage, radiation field extension and gestational age are the key factors influencing the risk of radiation-induced foetal morbidity [25]. During the first trimester and the early phase of the second trimester, the distance between the uterus and the radiation field is quite significant, and breast radiotherapy is considered feasible by some authors [43]. Nonetheless, only in case no other adjuvant treatment is indicated and after careful discussion of each individual case in a multidisciplinary team, it can be proposed to patients, keeping in mind that this indication is mainly based on theoretical assumptions and very limited experiences [11, 35].
42.4 Systemic Treatment
42.4.1 Chemotherapy
The indication for the use of chemotherapy in patients with BCP should follow standard recommendations as in the non-pregnant setting and should be based on both tumour biology and tumour stage; however, in this setting some specific issues should be taken into account including gestational age at diagnosis, expected date of delivery and the preferences of the patient and her family (◘ Fig. 42.1) [11, 25].
In patients with BCP, chemotherapy is contraindicated during the first trimester of gestation, while it can be safely administered in the second and third trimesters [11, 25] (◘ Tables 42.1 and 42.2).
The first trimester is the period of organogenesis which is characterized by high vulnerability to drugs with the possible occurrence of both spontaneous abortions and major congenital foetal malformations [11, 25]. According to the US National Toxicology Program Monograph, the overall rate of major malformations following exposure to chemotherapy during the first trimester was 14%; some chemotherapeutic agents (i.e. cyclophosphamide and 5-fluorouracil) have been associated with a higher risk of major malformations (18% and 31%, respectively) [44]. Additionally, the exposure to chemotherapy during the first trimester is associated with a 13% rate of spontaneous abortion, similar to the rate in healthy women [44]. Termination of pregnancy is not associated with improved maternal outcome [45]; however, for women with stage IV disease as well as for those with high-risk early-stage breast cancer diagnosed during the first trimester, termination of pregnancy can be considered to avoid delay in the initiation of cytotoxic therapy.
During the second and third trimesters, the administration of chemotherapy is associated with an overall 3% rate of major malformations [44], similar to the prevalence in the US general population [46]. Chemotherapy exposure during this period is associated with a rate of stillbirths of approximately 2% [44], slightly higher than in the US general population (0.3–0.4%) [47]. Therefore, it can be concluded that the use of chemotherapy during the second and third trimesters is feasible but can be associated with an increased number of obstetric and foetal complications (i.e. intrauterine growth restriction) [11, 25]. Prematurity is associated with impaired cognitive development [48, 49]; hence, prematurity should be avoided and, whenever possible, the goal is to target full-term delivery [11, 25].
Anthracycline-based or anthracycline/taxane-based chemotherapy regimens are standard of care for the treatment of breast cancer [50, 51] and should be recommended also in patients with BCP during the second and third trimesters [11, 25] (◘ Table 42.1).
Anthracyclines are the most studied chemotherapy compounds during pregnancy, with more than 400 women with BCP treated with these regimens [52]. Hence, anthracycline-based chemotherapy should be considered as the first choice [11, 25]. In non-pregnant breast cancer patients, the addition of 5-fluorouracil to anthracycline and cyclophosphamide has been shown to be associated with no survival benefit but increased toxicity [53]; hence, the combination of doxorubicin or epirubicin and cyclophosphamide (i.e. AC or EC) should be considered the preferred option also in women with BCP [11, 25] (◘ Table 42.1).
Clinical experience with the use of taxanes in patients with BCP is more limited. Docetaxel and paclitaxel are substrates for the placental P-glycoprotein transporter that seems to reduce the amount of drug passing from the placenta into the foetus [54]. In baboon models, docetaxel administered to the mother was not detected in foetal plasma but only in amniotic fluid and foetal tissues at a very low level; paclitaxel showed transplacental transfer, but the levels of drug in foetal tissues were very low and were not detected in the brain or cerebral spinal fluid [55]. In these models, both paclitaxel and docetaxel were shown to persist for a long time in foetal tissues, leading to a low level but long exposure [55]. In women with BCP, a systematic review including 50 pregnancies with exposure to paclitaxel and docetaxel showed that taxanes were well tolerated during pregnancy with manageable toxicities [56]. Hence, when clinically indicated, the use of taxanes can be considered during pregnancy [11, 25] (◘ Table 42.1). Due to the better toxicity profile and no need for granulocyte colony-stimulating factors (G-CSF) nor premedication with high-dose steroids, weekly paclitaxel should be preferred in women with BCP [11, 25].
In patients with higher-risk breast cancer, dose-dense regimens lead to improved overall and disease-free survival [57, 58]. In women with BCP, only one small retrospective cohort study in ten patients evaluated the feasibility of dose-dense chemotherapy during pregnancy [59]. Although the study showed no increased risk of foetal or maternal complication, due to both the limited data available and the need of G-CSF support, dose-dense chemotherapy should not be used in women with BCP (◘ Table 42.1).
Regarding optimal drug dosing in pregnant patients, clinicians should be aware that the pharmacokinetics of some cytotoxic drugs (e.g. doxorubicin, epirubicin, docetaxel and paclitaxel) might be altered during pregnancy [60, 61]. However, the calculation of the correct dose in women with BCP should follow the same standard procedures applied as in non-pregnant patients [11, 25]. A priori dose reduction as well as increased doses and treatment intervals should be avoided [11, 25].
A 3-week interval between the last dose of chemotherapy and the expected date of delivery should be allowed to avoid delivery during the nadir period [11, 25]. Due to the possible occurrence of spontaneous delivery after week 34 of gestation, chemotherapy should be discontinued at week 34 of gestation [11, 25] (◘ Table 42.1). Weekly chemotherapy regimens (e.g. weekly epirubicin and weekly paclitaxel) have a lower risk of haematological toxicity and shorter nadir periods; hence, they might be considered as a valid treatment option in pregnant patients, particularly as single drug treatment in the metastatic setting [11, 25].
42.4.2 Anti-HER2 Agents
Trastuzumab, a humanized recombinant IgG1 monoclonal antibody, is approved for the treatment of breast cancer patients with HER2-positive disease in the neoadjuvant, adjuvant and metastatic settings.
The HER2 pathway has a crucial role in organogenesis for normal cardiac development and migration of neural crest cells and seems to be also involved in the early conception and implantation phases [62]. IgG antibodies can cross the placenta starting from the second trimester of pregnancy with a continued increase of passage from then on up to term [63]. No embryolethal or fetotoxic effects with the use of trastuzumab have been reported in animal studies so far [64].
In humans, around 34 breast cancer patients exposed to trastuzumab during pregnancy have been described [65]. In all of the five cases where trastuzumab was «intentionally» started during the second or third trimester, the pregnancy was complicated with oligohydramnios resulting in preterm delivery [66]. The remaining 29 cases became accidentally pregnant during trastuzumab treatment with consequent exposure during the first trimester [66, 67]. First-trimester exposure was not associated with pregnancy complications or foetal malformations, and no cases of oligohydramnios were described [66, 67].
Hence, in contrast to chemotherapy, trastuzumab exposure during the period of organogenesis (i.e. first trimester) seems not to be associated with congenital malformations while beyond the second trimester is likely to produce «on-target» effects with a high number of cases who developed oligohydramnios (i.e. trastuzumab targets the HER2 oncogene which is also expressed in the foetal kidneys, the organs responsible for producing the amniotic fluid) [65].
These findings should be used for counselling patients in daily practice: first, women of childbearing potential treated with trastuzumab should be advised to use effective contraception [65]. Second, for women who become accidentally pregnant while on trastuzumab, since a brief exposure during the first trimester does not seem to increase pregnancy or foetal risk, it is plausible to consider stopping the medication to allow the continuation of the pregnancy [65]. Finally, according to treatment guidelines, elective administration of trastuzumab should be avoided during pregnancy but should be postponed until after delivery [11, 25] (◘ Tables 42.1 and 42.2).
Other anti-HER2 monoclonal antibodies (i.e. pertuzumab and T-DM1) are currently approved for the treatment of HER2-positive breast cancer, but no cases in women with BCP have been reported so far [65]. Since pertuzumab is approved in combination with trastuzumab, from a clinical standpoint, a similar approach to that of trastuzumab would apply for pertuzumab [65].
T-DM1, an antibody drug conjugate composed of trastuzumab connected to the cytotoxic drug emtansine, is a large molecule; hence, its transfer via the placenta as well as its toxic effects are expected to mimic that of trastuzumab [65].
Until further data are available, pertuzumab and T-DM1 should not be used in pregnant patients [65] (◘ Tables 42.1 and 42.2).
Lapatinib, a dual tyrosine kinase inhibitor targeting the epidermal growth factor receptor (EGFR) and HER2, is approved for the treatment of metastatic HER2-positive breast cancer. Being a small molecule, lapatinib is expected to cross the placenta throughout the pregnancy [65]. In humans, there is only one reported case of lapatinib exposure during pregnancy in a patient with metastatic disease that became accidentally pregnant during treatment [68]. Lapatinib was stopped around week 14 of gestation: no pregnancy complications or foetal malformations were observed [68]. Due to the very limited data on its safety during pregnancy, lapatinib should be avoided in women with BCP [65] (◘ Tables 42.1 and 42.2).
42.4.3 Endocrine Therapy
According to the ASCO guidelines for adjuvant endocrine therapy in young women with breast cancer, patients at low risk of relapse (i.e. women with stage I disease not warranting chemotherapy) should receive tamoxifen alone [69]. On the contrary, patients at higher risk (i.e. women with stage II–III disease candidates to adjuvant cytotoxic therapy or some women with stage I–II breast cancers at higher risk of recurrence who might consider chemotherapy) should receive ovarian suppression in addition to either tamoxifen or an aromatase inhibitor [69]. Similar recommendations are suggested by the BCY2 Panel [32].
In women with BCP, endocrine therapy is contraindicated during pregnancy [11, 25] (◘ Tables 42.1 and 42.2). In fact, foetal malformations (i.e. craniofacial malformations and ambiguous genitalia) have been described in children with in utero exposure to tamoxifen [70, 71]. Hence, the use of endocrine agents should be postponed until after delivery [11, 25].
42.4.4 Supportive Care
Although the majority of supportive regimens can be safely administered during pregnancy [11], in patients with BCP, these therapies should be used only if strictly indicated.
Anthracycline-based chemotherapy is associated with a particularly high risk of developing nausea and vomiting. According to the European Society for Medical Oncology (ESMO), in non-pregnant patients receiving anthracycline-based regimens, a three-drug combination of a neurokinin (NK) 1 receptor antagonist (days 1–3), a 5-hydroxytryptamine-3 (5-HT3) receptor antagonist (day 1) and dexamethasone (day 1) should be recommended to prevent nausea and vomiting [72]. Updated recommendations from ASCO support the same three-drug combination in all non-pregnant patients who receive anthracycline plus cyclophosphamide: palonosetron is the preferred 5-HT3 receptor antagonist in this setting, and the oral combination of netupitant and palonosetron (NEPA) plus dexamethasone represents an additional treatment option in this scenario [73].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree