The chemoprevention of breast cancer using pharmacologic agents has had substantial clinical success. Randomized clinical trials evaluating selective estrogen-receptor modulators (SERMs) have shown that these agents reduce the incidence of breast cancer by up to 50% in healthy women at increased risk for the development of the disease. SERMs have been of particular value in women with biopsy-proven risk factors, including atypical hyperplasia or lobular carcinoma in situ of the breast. The agents of established value are important options for women today, and efforts are under way to identify additional more effective therapies.
Despite newer, more effective treatments and improvements in detection, there were more than 42,000 deaths from breast cancer in the United States in 2009, according to the American Cancer Society. Because breast cancer is a major health problem for all women, the concept of preventing the disease is an attractive addition to the established roles of screening and treatment. Prophylactic mastectomy is an effective approach that reduces the risk of developing a breast cancer by an estimated 90% or more. However, it is a drastic and irreversible choice that is perhaps best reserved for women with high risk of the disease, such as those with known deleterious BRCA1 or 2 mutations. This article focuses on nonsurgical approaches to reducing breast cancer risk.
Some of the risk factors associated with breast cancer are modifiable, such as postmenopausal obesity, alcohol intake, and dietary fat. Reducing weight, exercising, low-fat diets, and moderation of alcohol intake are healthy behaviors. Such lifestyle modifications for breast cancer risk reduction remain an active area of research but are not yet of established value. The factors that place a woman at greatest risk (gender, age, and family history) are not amenable to lifestyle modifications.
Hong and Sporn have defined chemoprevention as “the use of pharmacologic or natural agents that inhibit the development of invasive breast cancer either by blocking the DNA damage that initiates carcinogenesis or by arresting or reversing the progression of premalignant cells in which such damage has already occurred.”
Selected estrogen receptor modulators
Although the precise mechanism or mechanisms that cause a breast cancer are not fully established, hormones play a significant role in a large percentage of cases, and current chemoprevention strategies have targeted hormonally responsive breast cancers. The selective estrogen receptor modulators (SERMs) are the group of agents that have been evaluated the most, and the best known of these drugs is tamoxifen. Tamoxifen is a well-established adjuvant therapy for receptor-positive breast cancer that has been shown repeatedly to reduce the risk of recurrence and death from this disease. In the large trials that showed tamoxifen to be an effective adjuvant treatment, there was also a substantial reduction in new primary cancers of the opposite breast. The duration of tamoxifen therapy in these studies varied from 1 to 5 years, but the reduction in opposite breast cancer persisted through at least 15 years of follow-up, showing this to be a durable, if not lifelong, benefit.
Tamoxifen chemoprevention trials
The findings from the adjuvant trials, combined with extensive laboratory data documenting the potential of tamoxifen as a chemoprevention agent, led to the initial randomized breast cancer chemoprevention trials. The largest of these trials was the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial, P-1, which randomized more than 13,000 healthy women from centers in North America to receive 20 mg of tamoxifen daily or placebo for a 5-year period ( Fig. 1 A).
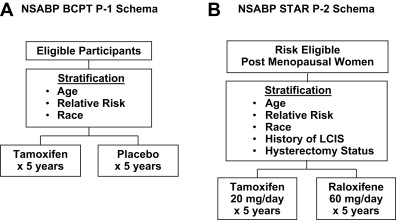
Eligible participants for the trial were at increased risk for breast cancer, which was defined as having 5-year projected invasive breast cancer risk of at least 1.66% as determined by the modified Gail model or having a history of lobular carcinoma in situ (LCIS) treated by local excision alone, or being at least 60 years of age. Women were not eligible for the trial if they had a history of deep vein thrombosis (DVT), a pulmonary embolus (PE), or if they had taken hormone therapy, oral contraceptives, or androgens within 3 months before random assignment into the trial. Estrogen or combinations of estrogen-progestin therapy were not permitted during the course of the study. The primary end point of the trial was the incidence of invasive breast cancer. Secondary end points included all other invasive cancers, noninvasive breast cancers, osteoporotic bone fracture, cardiac events, quality of life measurements, and death from any cause.
The initial results were published in 1998, with an average follow-up of approximately 4 years, and showed a highly significant 49% reduction in invasive breast cancer in favor of the tamoxifen-treated women (relative risk [RR] = 0.51, 95% confidence interval [CI] 0.39–0.66). The rate of noninvasive breast cancers (ductal carcinoma in situ [DCIS] and LCIS combined) was reduced by a similar magnitude. The reduction of invasive breast cancer appeared to be only in estrogen-receptor (ER)-positive cases, with a 69% reduction in incidence; there was no difference between the treatment groups in terms of the incidence of ER-negative disease.
Evaluation of secondary end points showed a 45% reduction in the number of osteoporotic fractures with tamoxifen (79 in the placebo group and 49 in the tamoxifen group). Tamoxifen was known to reduce blood lipids, but there was no reduction in the risk of myocardial infarction, severe angina, or acute ischemic syndrome noted in the tamoxifen-treated group compared with the placebo group. There were 28 cases of myocardial infarction in the placebo group and 39 in the tamoxifen-treated group (RR = 1.11; 95% CI 0.65–1.92). There were no significant statistical differences in the other 2 cardiac end points.
The toxicity associated with tamoxifen has been well documented in the treatment trials for invasive breast cancer and includes increases in the risk of endometrial cancer and thromboembolic events. Both of these toxicities were identified in the P-1 trial as well: the RR of endometrial adenocarcinoma was 2.53 (95% CI 1.35–4.97), with 15 cases occurring in the placebo group compared with 36 in the tamoxifen group. No excess of endometrial cancer was noted in the women younger than the age of 50 years, primarily a premenopausal group, at the time of random assignment. Thromboembolic events were also associated with tamoxifen use, and the magnitude of risk seems to be similar to that for postmenopausal women who take estrogen-replacement therapy. There was a 60% increase in the risk of DVT (RR = 1.60; 95% CI 0.91–2.86), with 22 cases occurring in the placebo group and 35 in the tamoxifen group. The risk of pulmonary embolism was also increased 3-fold (RR = 3.10; 95% CI 1.15–9.27), with 6 cases occurring in the placebo group and 18 in the tamoxifen group. There was a slight increase in strokes among women on tamoxifen, although it did not reach statistical significance. As with the endometrial cancer data, the increase in thromboembolic events appeared predominantly in women older than 50 years at the time they entered the trial.
The data for the use of tamoxifen in women with BRCA1or BRCA2 inherited mutations are limited, and definitive statements about the effectiveness of tamoxifen in such patients are not available. In the P-1 trial, King and colleagues identified only 18 women with inherited BRCA1 or BRCA2 mutations. Although tamoxifen reduced breast cancer incidence among BRCA2 carriers by 62%, this reduction was not statistically significant given the small numbers of patients out of the 13,000-plus women who entered the trial. Patients with BRCA2 frequently develop ER-positive breast cancers, although not uniformly. In the patients with BRCA1 noted in the P-1 trial, there was also no significant benefit from tamoxifen; there were numerically more cancers in the tamoxifen group. Narod and colleagues published a matched case-control study that reported significant protection from contralateral breast cancer in women with BRCA1 mutations who developed an invasive breast cancer and were treated with tamoxifen. A lesser effect was seen in women with BRCA2 mutations. That report and the P-1 data suggest that women with BRCA1 or BRCA2 mutations who have or will develop ER-positive breast cancer are potential candidates for tamoxifen therapy, but at the present time it is not possible to determine which women with BRCA1 or BRCA2 mutations will develop ER-positive disease.
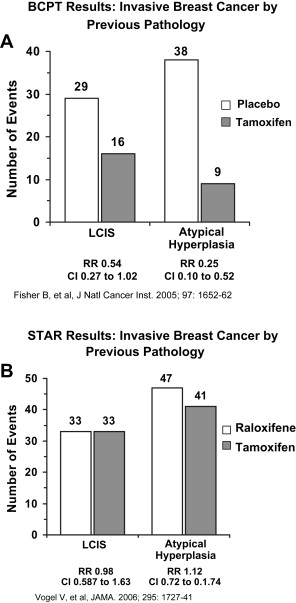
All the various subgroups of women in the P-1 trial appeared to benefit from the use of tamoxifen, including pre- and postmenopausal women as well as those with Gail scores ranging from 1.66% to more than 5%. The group that seems to have the greatest benefit is those women with a biopsy-proven history of LCIS or atypical hyperplasia. Such women are known to have a risk of subsequent invasive breast cancer that is 4- to 10-fold greater. In the P-1 trial, the annual breast cancer rate per 1000 women in the placebo arm with a history of LCIS at the time of entry was 12.99, and for those with a prior history of atypical hyperplasia, 10.11 ( Fig. 2 A). Both groups showed a substantial benefit from tamoxifen, with a 56% reduction in invasive breast cancer in women with LCIS and an 86% reduction in those with atypical hyperplasia.
Tamoxifen chemoprevention trials
The findings from the adjuvant trials, combined with extensive laboratory data documenting the potential of tamoxifen as a chemoprevention agent, led to the initial randomized breast cancer chemoprevention trials. The largest of these trials was the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial, P-1, which randomized more than 13,000 healthy women from centers in North America to receive 20 mg of tamoxifen daily or placebo for a 5-year period ( Fig. 1 A).
Eligible participants for the trial were at increased risk for breast cancer, which was defined as having 5-year projected invasive breast cancer risk of at least 1.66% as determined by the modified Gail model or having a history of lobular carcinoma in situ (LCIS) treated by local excision alone, or being at least 60 years of age. Women were not eligible for the trial if they had a history of deep vein thrombosis (DVT), a pulmonary embolus (PE), or if they had taken hormone therapy, oral contraceptives, or androgens within 3 months before random assignment into the trial. Estrogen or combinations of estrogen-progestin therapy were not permitted during the course of the study. The primary end point of the trial was the incidence of invasive breast cancer. Secondary end points included all other invasive cancers, noninvasive breast cancers, osteoporotic bone fracture, cardiac events, quality of life measurements, and death from any cause.
The initial results were published in 1998, with an average follow-up of approximately 4 years, and showed a highly significant 49% reduction in invasive breast cancer in favor of the tamoxifen-treated women (relative risk [RR] = 0.51, 95% confidence interval [CI] 0.39–0.66). The rate of noninvasive breast cancers (ductal carcinoma in situ [DCIS] and LCIS combined) was reduced by a similar magnitude. The reduction of invasive breast cancer appeared to be only in estrogen-receptor (ER)-positive cases, with a 69% reduction in incidence; there was no difference between the treatment groups in terms of the incidence of ER-negative disease.
Evaluation of secondary end points showed a 45% reduction in the number of osteoporotic fractures with tamoxifen (79 in the placebo group and 49 in the tamoxifen group). Tamoxifen was known to reduce blood lipids, but there was no reduction in the risk of myocardial infarction, severe angina, or acute ischemic syndrome noted in the tamoxifen-treated group compared with the placebo group. There were 28 cases of myocardial infarction in the placebo group and 39 in the tamoxifen-treated group (RR = 1.11; 95% CI 0.65–1.92). There were no significant statistical differences in the other 2 cardiac end points.
The toxicity associated with tamoxifen has been well documented in the treatment trials for invasive breast cancer and includes increases in the risk of endometrial cancer and thromboembolic events. Both of these toxicities were identified in the P-1 trial as well: the RR of endometrial adenocarcinoma was 2.53 (95% CI 1.35–4.97), with 15 cases occurring in the placebo group compared with 36 in the tamoxifen group. No excess of endometrial cancer was noted in the women younger than the age of 50 years, primarily a premenopausal group, at the time of random assignment. Thromboembolic events were also associated with tamoxifen use, and the magnitude of risk seems to be similar to that for postmenopausal women who take estrogen-replacement therapy. There was a 60% increase in the risk of DVT (RR = 1.60; 95% CI 0.91–2.86), with 22 cases occurring in the placebo group and 35 in the tamoxifen group. The risk of pulmonary embolism was also increased 3-fold (RR = 3.10; 95% CI 1.15–9.27), with 6 cases occurring in the placebo group and 18 in the tamoxifen group. There was a slight increase in strokes among women on tamoxifen, although it did not reach statistical significance. As with the endometrial cancer data, the increase in thromboembolic events appeared predominantly in women older than 50 years at the time they entered the trial.
The data for the use of tamoxifen in women with BRCA1or BRCA2 inherited mutations are limited, and definitive statements about the effectiveness of tamoxifen in such patients are not available. In the P-1 trial, King and colleagues identified only 18 women with inherited BRCA1 or BRCA2 mutations. Although tamoxifen reduced breast cancer incidence among BRCA2 carriers by 62%, this reduction was not statistically significant given the small numbers of patients out of the 13,000-plus women who entered the trial. Patients with BRCA2 frequently develop ER-positive breast cancers, although not uniformly. In the patients with BRCA1 noted in the P-1 trial, there was also no significant benefit from tamoxifen; there were numerically more cancers in the tamoxifen group. Narod and colleagues published a matched case-control study that reported significant protection from contralateral breast cancer in women with BRCA1 mutations who developed an invasive breast cancer and were treated with tamoxifen. A lesser effect was seen in women with BRCA2 mutations. That report and the P-1 data suggest that women with BRCA1 or BRCA2 mutations who have or will develop ER-positive breast cancer are potential candidates for tamoxifen therapy, but at the present time it is not possible to determine which women with BRCA1 or BRCA2 mutations will develop ER-positive disease.
All the various subgroups of women in the P-1 trial appeared to benefit from the use of tamoxifen, including pre- and postmenopausal women as well as those with Gail scores ranging from 1.66% to more than 5%. The group that seems to have the greatest benefit is those women with a biopsy-proven history of LCIS or atypical hyperplasia. Such women are known to have a risk of subsequent invasive breast cancer that is 4- to 10-fold greater. In the P-1 trial, the annual breast cancer rate per 1000 women in the placebo arm with a history of LCIS at the time of entry was 12.99, and for those with a prior history of atypical hyperplasia, 10.11 ( Fig. 2 A). Both groups showed a substantial benefit from tamoxifen, with a 56% reduction in invasive breast cancer in women with LCIS and an 86% reduction in those with atypical hyperplasia.