Breast Cancer
LOCALIZED BREAST CANCER
Neil M. Iyengar
Chau T. Dang
Epidemiology
Risk Factors
Strongest: Female gender, ↑ age
↑ estrogen: Early menarche, late menopause, late parity, or nulliparity (NEJM 2006;354:270); prolonged HRT (RR 1.24 after 5.6 y, JAMA 2003;289:3243); no ↑ risk w/OCP use (NEJM 2002;346:2025)
Benign breast conditions: ↑ risk: Proliferative features (ductal hyperplasia, papilloma, radial scar, sclerosing adenosis); atypia (atypical ductal or lobular hyperplasia); dense breast tissue (↓ mammographic Sn); no ↑ risk: Cysts, fibroadenoma, columnar changes (NEJM 2005;352:229)
Modified Gail model: Multivariate logistic regression model (www.cancer.gov/bcrisktool/)
Genetics (See Cancer Genetics Syndromes)
Screening
|
Mammography: 20-30% ↓ in breast CA mortality (<50 y smaller absolute benefit) (Lancet 2001;358:1340 & 2002;359:909; Annals 2002;137:347; Lancet 2006;368:2053)
Prevention
Tamoxifen: Risk-benefit: 43% 7-y risk reduction of invasive breast CA but ↑ DVT/PE, ↑ endometrial CA (NSABP P-1 (BCPT) JNCI 1998;90:1371 & Lancet 2002;360:817)
Raloxifene: 76% as effective as tamoxifen for risk reduction also ↓ vertebral fractures, ↑ risk of stroke, DVT/PE, cataracts but < tamoxifen (RUTH NEJM 2006;355:125), trend toward ↓ uterine CA (NSABP P-2 (STAR) JAMA 2006;295:2727)
Prophylactic bilateral mastectomy: 90% risk reduction for women at high risk (ie, BRCA 1/2, TP53, PTEN Mt)
Prophylactic bilateral salpingooophorectomy: ↓ risk of ovarian & breast CA (NEJM 2002;346:1609 & JAMA 2006;296:185)
Staging
Stage 0: Carcinoma in situ; Stage IA: T1 (tumor ≤ 2 cm), N0; Stage IB: T1N1mi (micrometastasis to LN > 0.2 mm but ≤ 2 mm); Stage IIA: T2 (tumor 2-5 cm), N0 or T1N1 (1-3 axillary nodes); Stage IIB: T2N1 or T3 (tumor >5 cm), N0; Stage IIIA: N2 (4-9 axillary nodes) or T3N1; Stage IIIB: T4 (direct extension to CW and/or skin or inflammatory); Stage IIIC: N3 (≥10 axillary nodes or any infraclavicular or ipsilateral supraclavicular nodes); Stage IV: Distant mets
Pathology
Molecular & Receptor classification (JCO 2010;28:1684)
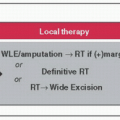
Local Management
Axillary LND for (1) >2 positive sentinel nodes, (2) clinically palpable nodes, or (3) positive axillary FNA or core bx
RT: Postmastectomy CW RT for ≥4 + LNs (1-3 + LNs send to radiation oncologist to decide), tumor >5 cm, Stage III, or + surgical margins: ↓ locoregional recurrence & ↑ survival (Lancet 2005;366:2087)
Adjuvant Management: When to Use Systemic Chemotherapy
Give chemotherapy to high risk – biologically (HER2 +, triple negative) or histologically (node-positive) for “most” pts to eradicate occult micrometastatic disease
Computer-based risk model: Adjuvant. Online (www.adjuvantonline.com) determines risk of recurrence based on age, comorbidities, tumor size/grade, hormone receptor status, nodal status; does not account for HER2 status (JCO 2001;19:980)
OncotypeDX: 21-gene signature RT-PCR assay to calculate RS – predicts 10-y distant recurrence rate; low (RS < 18) 6.8%; intermediate (RS 18-30) 14.3%; high (RS ≥ 31) 30.5%. Trials assessing chemo benefit in intermediate group based on Oncotype DX: TAILORx trial (completed) for node (−) & RxPONDER trial (ongoing) for postmenopausal pts w/1-3 (+) nodes
Pts w/ER+, LN−, & low RS derive min. benefit while pts w/high RS have improved DFS w/adjuvant chemotherapy (NSABP B20 JCO 2006;24:3726)
MammaPrint: 70-gene signature to catergorize into 2 groups – good or poor prognosis irrespective of ER status; study ongoing (MINDACT Lancet 2005;365:671)
Adjuvant Management: Chemotherapeutic Regimens
CMF (Cyclophosphamide, MTX, & fluorouracil) ↑ OS at 30-y follow-up (RR of D = 0.79, p = 0.04) compared to no chemotherapy after surgery (BMJ 2005;330:217); not recommended for high-risk disease
↑ DFS & OS when taxane added to anthra-based regimens (EBCTCG Lancet 2012;379:432; CALGB 9344 JCO 2003; NSABP B-28: JCO 2005;23:3686)
Standard Chemo Options for High-risk Pts:
ddAC → T (dose dense doxorubicin + C (AC) × 4 cycles → paclitaxel (T) × 4 cycles given q2wks w/g-CSF) > q3w AC → T (CALGB 9741 JCO 2003;21:1431)
TAC (docetaxel, doxorubicin, cyclophosphamide) > FAC (Fluorouracil, doxorubicin, cyclophosphamide) (BCIRG 001, NEJM 2005;352:2302)
FEC → D (fluorouracil, epirubicin, cyclophosphamide → docetaxel) (PACS 01, SABCS 2009)
FEC → wT (GEICAM 9906, JNCI 2008;100:805)
E → CMF (Epirubicin→CMF) (NEAT/BR9601, NEJM 2006;355:1851)
CEF (cyclophosphamide, epirubicin, fluorouracil) (MA 21, JCO 2010;28:77)
Nonanthra regimens
Adjuvant Management: Hormone Therapy for ER/PR Positive Disease
Premenopausal: ↓ breast CA mortality w/tam (Tamoxifen) – 31% ↓ × 5 y (NEJM 1998;339:1609) & 48% ↓ × 10 y & only slight ↑ DVT/PE & endometrial CA (ATLAS Lancet 2012)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree