Chapter 52 Bladder Cancer
Etiology and Epidemiology
Bladder cancer is the most common malignant disease involving the urinary system and the second most common genitourinary cancer. It is the fourth most common cancer among men and the ninth most common cancer among women. It is estimated that there will be 70,530 new cases and 14,680 bladder cancer deaths in 2010.1 There is little or no association between socioeconomic status and bladder cancer.2
Internationally, incidence rates of bladder cancer vary almost tenfold. The highest rates occur in western Europe and North America; relatively low rates are found in eastern Europe and several areas of Asia (i.e., China, India, the Philippines).3,4
Cancer of the bladder occurs primarily in older men, with a median age at diagnosis of 69 years. Two thirds of cases occur among persons aged 65 years and older. The incidence rate among African-American and Hispanic men is about 50% of that among whites. The male to female ratio is at least 3 : 1, approaching 4 : 1 in whites.5
From the period 1985 to 2005, the incidence rates in the United States increased by over 50%; from 1975 to 1996, the 5-year survival rate increased from 75% to 81%.1 The incidence of locally advanced and metastatic bladder cancer remained virtually constant.6
Risk Factors
Cigarette smoking increases the risk of bladder cancer twofold to threefold. Risk estimates for moderate to heavy smokers range from 2 to 10 compared with nonsmokers.7–11 Some studies indicate a reduction in risk by over 30% within the first 1 to 4 years after cessation of smoking, and over 60% 25 years after cessation, but even after 25 years the risk had not reached the background level of never smokers.9,11 Smoking cessation may decrease recurrence rates for patients with non–muscle-invasive disease.12 Exposure to secondhand smoke in women may be a risk factor for the development of bladder cancer.13
In the 1950s, occupational exposures to aromatic amines in the British dyestuff and rubber industries were found to be associated with bladder cancer.14,15 The mean time from start of exposure to death was 25 years, with the greatest risk for workers who started before age 25 years. Workers in the leather industry are at increased risk for bladder cancer, but the carcinogen has not yet been identified.16,17 The increased risk for painters seems to be due to many known carcinogens in paint such as benzidine, polychlorinated biphenyls, formaldehyde, asbestos, and solvents such as benzene, dioxane, and methylene chloride.18 Drivers of trucks, buses, or taxicabs are suspected to have an increased exposure to exhaust emissions containing polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs.19
It was first recognized in the 1970s that chlorination of drinking water generates a wide array of halogenated organic compounds. A number of epidemiologic studies investigated the possible association between exposure to these compounds and cancer. A 1992 meta-analysis of these studies found a significant association between exposure to chlorination by-products and bladder cancer.20 Several studies published subsequently supported this conclusion.21,22 High levels of arsenic in drinking water have also been linked to the subsequent development of bladder cancer.23
Aromatic amines are detoxified by N-acetylation. The N-acetyltransferase enzyme in the liver is polymorphic, displaying a slow and a rapid acetylator phenotype. The allele that confers a slow acetylator phenotype is assumed to be associated with an increased risk of bladder cancer (relative risk, 1.1 to 1.9).24
There are several other well-documented risk factors. Exposure to S. haematobium infection is associated with a predominantly increased risk of squamous cell carcinoma of the bladder.25 Heavy consumption of phenacetin-containing analgesics was linked to cancer of the renal pelvis, ureter, and bladder.26 Cyclophosphamide, an alkylating agent, and pelvic radiation have also been linked to the risk of bladder cancer.27–29 Other risk factors include low total daily fluid intake, chronic cystitis, and consumption of Chinese herbs containing aristolochic acid.30,31
Prevention and Early Detection
Primary prevention involves identification and avoidance of cancer-causing factors.32 In public health terms, avoidance and cessation of cigarette smoking is the most effective means available for the prevention of bladder cancer. Decreasing exposure to workplace carcinogens and increasing fluid intake are other useful approaches.
Secondary prevention involves screening individuals at high risk for a particular cancer with the goal of early detection and treatment. Urinary cytologic testing as a screening method has a low cost and low risk and has excellent specificity (>98%) but relatively poor sensitivity, particularly for low-grade tumors (overall sensitivity, 34%, with sensitivities for grade 1, 2, and 3 tumors of 12%, 26%, and 64%, respectively).33 Furthermore, the prevalence of preclinical bladder cancer is probably too low in the general population for large-scale screening programs to be rewarding. Testing for asymptomatic hematuria does not appear to be a useful method for screening. A single test has a low sensitivity. Repeat testing at short intervals, such as weeks, has been advocated but yields unacceptably high rates of false-positive results.34,35
Chemoprevention involves the administration of a natural or synthetic agent to healthy people (primary) or patients with precancerous conditions who are at high risk for bladder cancer (secondary) to prevent or retard the development or progression of cancer. Retinoids (vitamin A derivatives) as well as high-dose multivitamins, pyridoxine (vitamin B6), alpha-tocopherol (vitamin E), ascorbic acid, polyamine synthesis inhibitors, polyphenols (green tea extract), COX-2 inhibitors (celecoxib), EGFR tyrosine kinase inhibitors (erlotinib), and oltipraz have been or are under investigation as potential chemopreventive agents,36–46 but nothing definitive has been established at present. Additional studies are under way.
Pathology and Pathways Of Spread
In T2 lesions, muscle invasion is present and the probability of nodal and distant spread is increased. T2 disease is divided into superficial (T2a) or deep (T2b) invasion.47
TCC is often multifocal both in place and in time (polychronotropism). This is a striking characteristic of this disease, but the etiology is uncertain. Two of the most commonly held explanatory theories are a genetic field defect with multiple new tumors spontaneously arising or, alternatively, the local reimplantation of tumor cells (local metastasis). Evidence strongly suggests that tumor reimplantation or submucosal migration is the predominant mechanism. Multifocal tumors as well as upper tract and lower tract lesions arising in one individual demonstrate clonality.48
External and internal iliac chains as well as perivesical lymph nodes are first-echelon lymph nodes. Common iliac, para-aortic, and paracaval lymph nodes are considered second-echelon lymph nodes. Lymph node sampling in patients undergoing cystectomy should include excision of 12 or more lymph nodes.47
Molecular Biology
Newly discovered cellular mechanisms and their associated proteins have become subjects of intense interest. This is partly for what they tell us about the biology of a tumor but also because of their potential role as prognostic markers and/or therapeutic targets. It has long been recognized that invasive bladder tumors are extremely heterogeneous in nature, despite almost always having high-grade, transitional cell morphologic characteristics because of complex genetic and epigenetic interactions that play a direct role in the initiation and progression of TCC. When the range of genetic alterations is considered, deletions (e.g., loss of tumor suppressor genes) generally arise in early-stage disease, whereas gains and amplifications (e.g., activation of oncogenes) may be more frequent in advanced stages of disease. Genetic factors may also modify the risk associated with exogenous agents through activation or detoxification of potential carcinogens. The search to understand the heterogeneity and use it to therapeutic advantage is only just beginning. At the moment, the best prognostic indicators we have in bladder cancer are clinical ones of stage, grade, tumor size, unifocality or multifocality, and the presence or absence of either hydronephrosis or lymph node involvement (discussed further later in this chapter). No molecular biomarker yet comes close to improving upon these clinical markers, but they likely will in the next decade.49 This will probably involve a combination of multiple independent markers, and an individual tumor’s gene expression pattern is an intriguing area of research.
Apoptosis and Cell Cycle Receptors
These receptors provide malignant cells with an evolutionary selective advantage. These include cellular immortalization, escape from G1 checkpoint control, loss of DNA damage checkpoints (G2/M), loss of normal DNA repair mechanisms, loss of apoptotic response, and the development of invasive capabilities.50–52 The pRB and TP53 pathways, which involve several common molecules, provide G1 checkpoint control and regulate the response to DNA damage. Though the RB gene itself has mutated in only a few specific cancers, it is thought that abnormalities of its function are far more common and that transitional cell bladder cancer represents one of the abnormalities.51,53–55 Disruption of the gene’s function is required to overcome the restraint it imposes on cell cycle progression. The proapoptotic tumor suppressor gene eliminates cells with abnormalities in their RB pathways. Tumors therefore commonly have abnormalities in p16 and p14ARF, and abnormalities at these loci may also be required to overcome the checkpoints that protect the cell from aberrant stimuli. TP53 gene mutations, which may be detected by nuclear immunohistochemical testing, are present in over half of bladder tumors and appear to be related to the progression of bladder cancer and to the outcome following treatment.53,56,57–59 A meta-analysis of 168 studies assessing TP53 overexpression showed a weak, although significant, association with the overall risk of recurrence and mortality.60 Cooperative group trials are under way to test the role of three cycles of combination chemotherapy in patients with negative nodes but mutated TP53 genes. Work from the Radiation Therapy Oncology Group (RTOG) on patients who were treated with chemotherapy and irradiation has not, however, shown any clear association between pRB, p16, or TP53 status and outcome.61 Clearly, the implication of the expression of these markers will vary according to the treatment planned.
Other genes regulating apoptotic function or involved in the initiation of bladder carcinogenesis and its progression have been studied, such as the BAX, BCL2, and p21 genes, survivin, and matrix metalloproteinases.62,63,64,65–69 In examining a large database containing bladder cancer of different stages all treated by a variety of methods, it did seem that those who had BAX or CD40L tumors lived longer. BCL2 positivity appeared to predict worse survival times. The caveat, however, is that most of these were not prospective studies balanced for other prognostic variables.
Researchers from Germany have suggested that one does not have to analyze the molecular pathways relating to apoptosis to predict a response; a measure of the baseline spontaneous apoptotic rate of the tumor may be easier and quite sufficient. The inability to undergo spontaneous apoptosis whether due to overexpression of BCL2 or survivin predicts local failure after irradiation and chemotherapy.70
The RAS family of transforming genes is often overexpressed in bladder cancer. Their presence may be related to the original development of the tumor. In a mouse model, farnesyltransferase inhibitors that inhibit the post-translational modifications of HRAS worked synergistically with irradiation in reducing tumor recurrence.71
Growth Factor Receptors
Both EGFR and HER2 are commonly overexpressed in urothelial tumors. Numerous immunohistochemical studies have investigated the expression of HER2 in bladder cancer and have found overexpression in 40% to 80% of tumors.60,72–75 There are, however, conflicting data on the relationship between the expression of these receptors with response to treatment and clinical outcome. A recent RTOG study evaluated the clinical relevance of EGFR and HER2 expression as measured by immunohistochemical staining on slides from 73 patients treated on four of the RTOG bladder preservation trials. Results pointed to a more favorable prognosis in patients with overexpression of EGFR but a lower complete response rate in patients with TCC with overexpression of HER2.61 EGFR positivity correlated with an improved overall survival (OS) rate (p = .044), disease-specific survival rate (p = .042), and survival rate with an intact bladder (p = .021). A trend toward a decreased incidence of distant metastases was also associated with EGFR expression. On multivariate analysis, adding tumor stage, tumor grade, whether or not a visibly complete TURBT was done, and patient age to the model, EGFR positivity remained significantly associated with an improved disease-specific survival rate. HER2 overexpression correlated with reduced complete response rates to chemoradiation (50% vs. 81%; p = .026), which remained significant on multivariate analysis. Unlike findings in other studies, TP53 and p16 had no prognostic significance. These patients had all been treated with chemotherapy and irradiation, and it may be that EGFR has a more direct involvement in improving the response of TCC to these agents than it does other tumors. It will be crucial to elucidate this relationship clearly because a growth factor receptor may be either a favorable or an unfavorable marker, depending on the proposed therapy. The RTOG data would suggest that targeting the HER2 pathway may be more profitable than EGFR in the initial therapy of muscle-invasive bladder cancer. There is certainly experimental evidence that HER2 has an antiapoptotic effect and that tumor radiosensitization can result from targeting this mechanism.76 An ongoing RTOG trial (05-24) is evaluating the addition of trastuzumab to paclitaxel and daily radiation therapy following TURBT in the treatment of noncystectomy candidates with muscle-invasive bladder cancer.
Recent data suggest that the presence of mutations in fibroblast growth factor receptor 3 (FGFR3) identifies a subgroup of patients with a favorable prognosis.77 Further investigation into the potential diagnostic and therapeutic implications of such findings is warranted and may lead to the rational development of targeted therapies.
Hypoxia
Hypoxia is well recognized to be strongly associated with both irradiation and chemotherapy resistance. It results in the up-regulation of genes that facilitate anaerobic metabolism and promote tumor vascularization. One such hypoxia-inducible factor is carbonic anhydrase IX (CA IX). It appears to be useful as a surrogate marker of hypoxia within bladder tumors. Several studies have shown that the majority of bladder tumors express this marker, although it appears, strangely, to be more strongly expressed in superficial tumors than invasive tumors. Evidence on its role as a prognostic marker has been conflicting.78,79
Clinical Presentation, Patient Evaluation, and Staging
Clinical Presentation
Painless, gross, and episodic hematuria is the most frequent presenting manifestation of bladder cancer (80%), typically present through the entire course of micturition. It also may be associated with irritative symptoms (e.g., dysuria, frequency, or urgency), which can occur in up to one third of patients and are suggestive of bladder CIS. Ten percent to 20% of patients presenting with gross hematuria are diagnosed with bladder cancer.80,81 Some asymptomatic patients proven to have bladder cancer are found to have microscopic hematuria or, less frequently, pyuria. Bladder tumors can cause strangury (pain on straining to urinate), urinary retention, ureteral obstruction, sepsis, and, rarely, life-threatening hematuria. Regional pelvic disease may cause flank pain because of nerve invasion, edema, and ureteral obstruction. The signs and symptoms of bone, liver, pulmonary, and central nervous system metastases may be present if disease has disseminated.
Workup of Patients with Hematuria or Suspected Bladder Tumors
Endoscopy
The endoscopic evaluation forms the mainstay of diagnosis and staging. Initially, an outpatient cystoscopic examination with biopsy is performed, and urine is obtained for cytologic studies. Cystoscopy provides direct visualization of the bladder and facilitates biopsy of the bladder. The number, size, shape, and location of tumors as well as the appearance of the surrounding mucosa, urethra, and urethral orifice are documented. The tumor size (<2 cm, >5 cm) and shape (papillary or flat) and the presence of associated Tcis lesions are of predictive importance. Using an illustrative bladder map, the location, number, size, and characteristics (papillary vs. sessile) of the bladder tumor as well as the presence or absence of CIS should be noted. Fluorescence cystoscopy after intravesical instillation of a photoactive substance (e.g., a porphyrin such as 5-aminolevulinic acid [5-ALA]) that preferentially accumulates in neoplastic tissue may be more effective than white light cystoscopy. Randomized trials have shown that fluorescence endoscopy detects more tumors (particularly CIS), and this improved tumor identification may result in better patient management.82 This procedure has been approved in Europe but remains investigational in the United States.
Urinary Cytology
Urinary cytologic findings should be used to complement the findings of cystoscopy. It is extremely valuable for the diagnosis of high-grade TCCs, especially CIS that might be difficult for the endoscopist to visualize, and upper tract malignant disease. The presence of high-grade TCC in the cytologic specimen in patients with low-grade papillary TCC suggests either unrecognized CIS or, less commonly, high-grade disease in the upper urinary tracts.83,84 For the diagnosis of low-grade papillary tumors, cytologic testing is not sensitive, because the appearance of grade 1 TCC is identical to that of normal urethral cells.33
Cytology has been regarded as the gold standard for noninvasive screening of urine for bladder cancer. It has a sensitivity of around 40% to 60%, with a specificity in excess of 98%.33 Cytology is illustrative of the problems of noninvasive screening. Poorly differentiated tumors have a false-negative detection rate of 20%, whereas well-differentiated tumors have a false-negative detection rate of up to 80%.
Multiple urine-based biomarkers have been developed because of the low sensitivity of cytologic testing, and they perhaps benefit from the lack of subjectivity by the person reading the test.85–90 These tests identify proteins in urine, detect cellular antigens by immunohistochemical or cytochemical testing, or use fluorescence in situ hybridization (FISH) to identify genetic alterations associated with bladder cancer. Markers include nuclear matrix proteins (e.g., NMP22), fibrin/fibrinogen degradation products (FDPs), cytokeratins,8,18–20 hyaluronic acid, urinary bladder tumor antigen (BTA), basic fetoprotein, survivin, telomerase, microsatellite DNA, and chromosomal abnormalities (3, 7, 17, 9p21). These approaches have demonstrated a higher sensitivity than urinary cytologic testing but a lower specificity. None of these markers, however, have sufficient sensitivity (especially for low-grade small tumors) and diagnostic reliability to replace cystoscopy, and their clinical use has not been recommended by consensus panels. Additional trials are needed to evaluate these newer techniques and their clinical effectiveness and cost-effectiveness as they are integrated into patient management.
Imaging
Although fewer than 60% of bladder cancers are seen on intravenous urography, this imaging modality provides valuable information about the status of the upper urinary tract.91 The presence of ureteral obstruction or a nonfunctioning kidney predicts muscle-invasive disease in 90% of cases.92 Concomitant or subsequent upper urinary tract tumors are found in 2% to 5% of patients. CT urography has replaced IVP as the procedure of choice, though IVP remains an appropriate alternative when CT is not available.
Evaluation of a patient with potential muscle-invading bladder cancer should include CT scanning of the abdomen and pelvis (with and without contrast and with delayed images). Although it is not sensitive in reliably describing the depth of invasion or lymph node involvement, it can be helpful in determining extravesical extension and the planning for subsequent treatment. Notably, the detection of lymph node metastases with CT imaging is poor (high false-negative rate). About 20% to 30% of patients with node-negative disease by CT scanning have positive nodes at the time of cystectomy with lymphadenectomy.93,94
Gadolinium-enhanced MRI studies may prove useful in distinguishing superficial from invasive disease and intravesical from extravesical tumors.95–99 Lymphangiography using iron nanoparticles and MRI might be particularly useful in detecting early nodal metastases.100 Positron emission tomography (PET) scanning has limited value. All bladder-specific imaging should be performed before TURBT is attempted to avoid postsurgical edema.
Staging
Stage is the most important independent prognostic variable for progression and OS for invasive bladder cancer. The basis for the major staging systems is the classic clinicopathologic study by Jewett and Strong,101 which correlates the probability of regional lymph node and distant metastases with the depth of invasion of the primary bladder tumor. The TNM (tumor, node, metastasis) system of 2010102 is shown in Table 52-1 and Figure 52-1. Of note, CT and MRI are not used for staging of the primary tumor in the TNM system. If lymphadenectomy is performed, sampling should include excision of an average of more than 12 lymph nodes.
TABLE 52-1 AJCC 2010 TNM Bladder Cancer Staging
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Ta | Noninvasive papillary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor invades the lamina propria (subepithelial connective tissue) but not beyond |
| T2 | Tumor invades the muscularis propria |
| pT2a | Tumor invades superficial muscle (inner half) |
| pT2b | Tumor invades deep muscle (outer half) |
| T3 | Tumor invades perivesical tissue |
| pT3a | Microscopically |
| pT3b | Macroscopically (extravesical mass) |
| T4 | Tumor invades any of the following: prostate, seminal vesicles, uterus, vagina, pelvic or abdominal wall |
| T4a | Tumor invades prostatic stroma, uterus, vagina |
| T4b | Tumor invades pelvic or abdominal wall |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Single regional lymph node metastasis in the true pelvis (hypogastric, obturator, external iliac, or presacral) |
| N2 | Multiple regional lymph node metastases in the true pelvis (hypogastric, obturator, external iliac, or presacral) |
| N3 | Lymph node metastasis to the common iliac region |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
In clinical practice, the correlation between depth of invasion, based on cystoscopic evaluation, and the final cystectomy specimen is only 50% to 60%. That is to say that 40% to 50% of patients undergo pathologic upstaging.103 The accuracy of the determination of degree of muscle infiltration is, therefore, modest at best. The clinical staging system as proposed by the Union Internationale Contre le Cancer was often more practical. Cancer without residual induration after TURBT was staged as T2 and cancer with palpable residual induration as T3. The most important determination of treatment outcome seems to be whether the tumor is confined to the organ (≤T2) or has spread beyond it (≥T3). When comparing outcomes for different treatment modalities, it is very important to consider whether the disease was staged clinically or whether a cystectomy specimen was available for thorough pathologic staging.
Primary Therapy
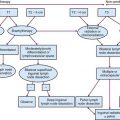
The most appropriate treatment algorithm for muscle-invading disease remains controversial. Although radical cystectomy has long been the standard treatment in the United States, organ-preserving regimens using irradiation with concurrent chemotherapy are emerging as viable alternatives in a subset of patients (Fig. 52-2). These two approaches appear to have similar survival rates in uncontrolled comparisons, but strong physician and patient preferences have so far prevented a randomized, controlled trial comparing these treatment options.
Superficial Bladder Cancer (Ta, T1, Tcis Disease)
Transurethral Resection (TURBT)
The majority (40% to 80%) of patients with superficial TCC will eventually experience recurrent disease after TURBT.104 Progression to invasive disease after TURBT alone occurs in 10% to 25% of patients and is closely associated with tumor type, stage, and grade.105,106 Other factors include multicentric disease, frequency of recurrence, tumor size, and the presence or absence of concomitant CIS.
The risk of progression is substantially higher for tumors of a higher grade or stage. Ta, G2 to G3 disease will progress to invasive TCC in 20% of cases at 5 years after treatment with TURBT alone. Nearly all patients (>70%) with T1 disease have high-grade tumors, and approximately 50% experience invasive disease at 5 years. In patients with T1 disease with CIS in the pathologic specimen, the rate of progression rises to 80% at 5 years.107,108
Intravesical Therapy
Bacille Calmette-Guérin
Several randomized, controlled trials compared TURBT alone with TURBT plus BCG.109–113 Those treated with BCG had significantly fewer recurrences at 12 months. For patients with Ta, G2 to G3, and T1 disease, adjuvant therapy with BCG reduces the rate of progression to 5% to 15% at 5 years. Nonetheless, 50% of patients with T1/CIS will experience muscle-invading cancer at 10 years. Progression rates to muscle invasion among patients who respond to BCG are 1% at 1 year, 5% at 3 years, and 15% at 5 years.
The positive impact on progression translates into a survival advantage.114,115 With long-term follow-up, adjuvant intravesical BCG improved survival rates to 88%, compared with 63% with TURBT alone, and reduced the cystectomy rates from 42% to 20%.114
Intravesical therapy is the first-line treatment for diffuse CIS. The average complete response rate of CIS to BCG is greater than 70% for more than 1 year.116 This treatment prevents subsequent disease in 60% of cases for up to 5 years and in 40% of cases for up to 10 years. Particularly in CIS of the prostatic urethra, BCG has spared many patients from having to undergo cystectomy. TURBT is recommended to stage the disease and to open the bladder neck to allow BCG to bathe the prostatic urethra. Low-grade superficial disease with frequent recurrence is also considered an indication for intravesical therapy.
One consequence of controlling the bladder component of disease in a patient with a TCC of the bladder is an increase in the frequency of extravesical relapses117; hence, careful follow-up is required. Relapses can develop at any point at which transitional epithelium is found: the renal pelvis, ureters, and urethra. The risk for an upper tract tumor is 20% at 5 years, 25% at 10 years, and 33% at 15 years. The risk of prostatic urethra and duct involvement at 5 years is 10% to 15% and at 10 years, 20%. If complete resection of tumors of the urethra cannot be obtained, patients are managed with cystectomy. Patients with positive cytologic testing and no obvious bladder tumor need careful monitoring of the upper tracts. Ureteroscopic resection and instillation of BCG through the renal pelvis is possible.
Other Agents
Although BCG is the treatment of choice for intravesical therapy, a variety of other agents have been investigated, such as mitomycin, doxorubicin, epirubicin, valrubicin, thiotepa, gemcitabine, interferon, and docetaxel. None has proved consistently superior to BCG,116,118 especially in cases of high-risk disease features (high grade, T1, Tis), though they may have a role in BCG failures and as an immediate single postoperative dose for patients at low risk of recurrence following TURBT. Novel approaches to decrease recurrence following initial treatment for non–-muscle-invasive bladder cancer include photodynamic therapy.
Role of Irradiation in High-Risk Superficial Bladder Cancer
It is well recognized that TURBT may clinically understage tumors as superficial when they have already invaded the muscle wall (≈20% to 30%). Radiation therapy, therefore, has an advantage in that it can reach tumor deposits too deep for instillation therapy. There have been no randomized trials comparing radiotherapy with current intravesical treatment options. The Dutch South Eastern Bladder Cancer Group has presented data in 121 patients with T1G3 cancers.119 EBRT with 50 Gy/25 fractions was one treatment option, and 17 patients received this. Though the numbers are limited, the treatment appeared to be as effective as intravesical BCG or mitomycin. Good results have also been achieved at the University of Rotterdam with interstitial implants.120 In this selective but prospective series, patients with single T1 tumors of less than 5 cm in diameter underwent TURBT and subsequent local irradiation of the tumor area in the bladder wall by an interstitial radium implant. The definitive local control rate was 82% and the 10-year OS was 76%. Another group in the Netherlands has published their results using a combination of TUR, EBRT at a dose of 3.5 Gy in 3 to 4 fractions, and interstitial radiotherapy at a mean implant dose of 55 Gy in patients with solitary T1G3 and T2 tumors.121 The local control rate was 70% in the whole patient population. The 5-year disease-specific survival (DSS) was 80% for stage T1 and 60% for T2a tumors. One randomized trial of radiation therapy alone (60 Gy in 30 fractions) in the management of high-grade T1 TCC of the bladder did not seem to indicate a benefit over observation for patients with unifocal disease or over BCG for those with multifocal disease or CIS.122
A large series of 141 patients with high-risk T1 cancers treated with either TURBT plus EBRT with or without platinum-based chemotherapy was conducted at the University of Erlangen.123,124 In this series, 88% of patients achieved a complete response; about 15% progressed to muscle-invasive disease after 5 years and 30% at 10 years, which compares favorably to most studies of TURBT and instillation therapy. DSS were 82% and 73% at 5 and 10 years. In more than 80% of survivors, the bladder was preserved, and 70% were “delighted” or “pleased” with their urinary function. These results suggest that irradiation may have a role to play in patients with high-grade or recurrent T1 lesions and may be attempted ahead of cystectomy on protocol or in place of cystectomy in those who are medically inoperable.
Muscle-Invading Bladder Cancer (T2-4 Disease)
Radical Cystectomy
Surgical Methods
Radical cystectomy entails the surgical removal of the bladder, adjacent organs, and regional lymph nodes. Although traditionally performed as an open procedure, laparoscopic or robotic radical cystectomy has been investigated as a less invasive alternative. In men, the bladder, prostate, seminal vesicles, proximal vas deferens, and proximal urethra, with a margin of adipose tissue and peritoneum, are resected en bloc. In women, the procedure involves an anterior pelvic exenteration to remove the bladder, urethra, uterus, fallopian tubes, ovaries, anterior vaginal wall, and surrounding fascia en bloc. The extent of pelvic lymph node dissection is an important predictor of outcome, and many centers have adopted an extended template dissection to include presacral and common iliac lymph nodes to the aortic bifurcation and often more proximal to the origin of the inferior mesenteric artery, in addition to pelvic lymph nodes distal to the common iliac bifurcation.125 Before cystectomy, grossly abnormal lymph nodes are sampled. In cases of metastases, cystectomy is aborted unless urinary diversion is required to relieve symptoms. Urinary diversion is usually accomplished through an ileal conduit opening onto the anterior abdominal wall through a urostoma.
Continent Urinary Tract Reconstruction
Cutaneous Urinary Reservoir
The two main options are reservoirs made from the colon and small bowel (Indiana pouch) or entirely from the small bowel (Kock pouch).126,127 The Indiana pouch consists of approximately 25 cm of right colon, often including some distal ileum, which is brought to the abdominal wall. It relies on the ileocecal valve for continence. Patients catheterize the pouch on an average of four to six times per day.
Orthotopic Diversion
A neobladder is constructed from ileum, ileocolon, or sigmoid colon and anastomosed to the native urethra as opposed to the abdominal wall. This permits the patient to void in a relatively normal fashion per the urethra. The Mainz pouch (neobladder) consists of an ileocecal segment, which is detubularized and anastomosed to the urethra.128 The Hautmann pouch consists of a W-shaped ileoneobladder, in which 60 cm of ileum is configured into a sphere.129
The choice of urinary diversion is based upon patient and surgeon preferences and may be influenced by the extent of cancer. Orthotopic bladders are contraindicated when the tumor involves the urethra. Orthotopic neobladders are becoming increasingly common in patients undergoing cystectomy but have been predominantly used in men. They are less common in women because of the technical difficulties in maintaining continence and the risk of urethral recurrence given the shorter length of the female urethra.130
Morbidity of Cystectomy
Operative mortality from cystectomy in modern series ranges from 1% to 3%, with postoperative complication rates ranging from 15% to 64% and a readmission rate of 26%.131,132,133,134 Complications include hemorrhage, rectal injury, deep venous thrombosis, pulmonary embolus, pelvic abscess, sepsis from urine or bowel leak, wound infection/dehiscence, and small bowel obstruction. The frequency of complications appears to be related to surgeon experience, hospital volume, age, and presence of comorbidities.
The need for urinary diversion and loss of sexual function in patients undergoing radical cystectomy are the most serious side effects and may impair the quality of life dramatically. In women, loss of sexual function occurs secondary to subtotal vaginectomy and in men, secondary to prostatectomy with loss of vascular supply or nerves serving penile erection. Newer nerve-sparing techniques for men allow for approximately 50% of selected patients to regain their erectile potency.135
Cancer Outcome after Cystectomy
Contemporary large series provide the best data on outcome after radical cystectomy.136,137,138,139 The University of Southern California reported on 633 patients undergoing radical cystectomy with pathologic categories pT2 to T4a with an actuarial OS at 5 years of 48% and at 10 years of 32%.136 The series from the Memorial Sloan-Kettering Cancer Center showed that in 184 patients with tumors of pT2 to T4, 5-year OS was 36%. Five-year OS for all 269 patients with pathologic categories ranging from pT0 to pT4 was 45%.137
The presence or absence of lymph node disease and its extent and the lymph node density (ratio of number of positive lymph nodes to the number of lymph nodes removed) are also significant determinants of survival rates.140 The estimated probability of 5-year OS of patients with nodal metastases is 29%; with one to five nodes positive, 35%, and with six or more nodes positive, 17%.141 The incidence of positive lymph nodes varies according to pathologic tumor stage. In cystectomy series, it ranged from 0% to 6% for superficial disease, 18% to 22% for muscle-invasive disease (pT2, 6% to 20%; pT3a, 30% to 31%), 30% to 64% for disease invading the perivesical fat (pT3b), and 45% to 59% for disease invading adjacent organs (pT4).142,143,144
Other factors that may influence the outcome include the quality of surgery, margin status, presence of lymphovascular invasion, and marker status (TP53, p21, pRB, p16). Nomograms that consider multiple prognostic factors such as stage, nodal status, tumor grade and histologic type, lymphovascular invasion, and patient age have been developed.145
Irradiation as an Adjunct to Cystectomy
Preoperative Radiation Therapy
The recognition that moderate-dose irradiation (20 to 50 Gy) may reduce the volume of gross disease and eradicate microscopic TCC led to its frequent use before cystectomy in the 1970s.146,147 In nonrandomized studies, these doses were found to reduce the frequency of metastases in the lymphadenectomy specimens by approximately 50%.148 By the 1980s, improvements in the technique of radical cystectomy, together with the introduction of more extensive therapeutic lymphadenectomies, made many question the need for radiation therapy.149 It has been argued that irradiation delays the time to definitive surgery, increases its morbidity, and may compromise the surgeon’s ability to perform continent urinary diversions. The question was addressed by an intergroup trial reported in 1997150 in which 140 patients with invasive or recurrent superficial bladder cancers were randomized to receive cystectomy alone or plus preoperative irradiation (20 Gy in five fractions). There was no significant survival advantage to either group at 5 years. The trial was small, the doses were low, and the predominant pattern of failure was distant, all factors that would make it practically impossible to detect any advantage to irradiation. Parsons and Million151 did, however, demonstrate an apparent survival advantage for patients with clinical T3 tumors who received preoperative irradiation.
Cole and colleagues152 reported on 133 patients with T3b disease treated at the M.D. Anderson Cancer Center (MDACC). This retrospective review documented that when pelvic wall disease recurs following modern radical cystectomy, it is usually only in patients who had clinical stage T3b or T4 tumors. The researchers report a 28% incidence of pelvic recurrence in stage T3b patients treated by radical cystectomy, with or without multidrug chemotherapy. When stage T3b patients were treated with preoperative radiation therapy (which this institution used extensively prior to 1980), the pelvic recurrence rate was only 10%, however. Before 1983, all patients received 50-Gy preoperative irradiation, followed 4 weeks later by cystectomy. The actuarial 5-year pelvic control rate was 91%. After 1983, radiation therapy was discontinued, and subsequent pelvic control rates have fallen to 73% despite improvements in surgical technique and staging and the addition of systemic chemotherapy. A survival decrement (52% to 42%) was also seen. The extent to which patient selection factors contributed to this observed difference is unknown. Others have reported lower rates of pelvic recurrence using cystectomy alone for this subset of patients, and it therefore remains uncertain as to whether these findings are widely applicable.149
The morbidity of preoperative irradiation is small. Whitmore148 reported comparable rates of wound healing and perioperative mortality for patients who received and those who did not receive irradiation before cystectomy. There is limited experience in the creation of continent urinary diversions after preoperative irradiation, although Housset and colleagues153 report that it is possible after the delivery of 44 Gy (24 Gy delivered in eight fractions over 17 days according to a modified bifractionated split-course schedule [3-Gy fractions twice daily on days 1, 3, 15, and 17] followed by a 20-Gy boost [2.5-Gy fractions twice daily on days 64, 66, 78, and 80]).
TCCs of the bladder have a high propensity to autotransplantation. Iatrogenic wound seeding after cystotomy was commonly reported by those who perfomed partial cystectomies or bladder brachytherapy. Low-dose preoperative irradiation (8.5 to 20 Gy) profoundly reduced the incidence of this problem.154 Low-dose irradiation is still occasionally given in the United States prior to a partial cystectomy. The partial cystectomy is, however, not commonly performed, because it is limited to small, unifocal tumors of the dome, an unusual location.
Postoperative Radiation Therapy
When the cystectomy specimen shows extensive extravesical disease or positive surgical margins, the risk for pelvic recurrence is high. Postoperative radiation therapy has been evaluated in only one randomized study. The National Cancer Institute of Egypt reported on 236 patients with T3 to T4 tumors (68% were SCCs) who received either no adjuvant therapy or postoperative irradiation (using three daily fractions of 1.25 Gy each, with 3 hours between fractions, up to a total dose of 37.5 Gy in 12 days [75 patients]; or conventional fractionation for a total dose of 50 Gy over 5 weeks [78 patients]).155 The 5-year rates of disease-free survival (DFS) (44% to 49% vs. 25%) and local control (87% to 93% vs. 50%) were improved and the incidence of pelvic recurrence was significantly reduced in the groups receiving postoperative irradiation. Whether this finding is as applicable to TCCs as to squamous cell tumors remains an unanswered question. Today, patients with such extensive disease commonly receive adjuvant chemotherapy, although the ability of this modality to prevent local recurrence has been called into question by Cole and colleagues.152 Radiation therapy rarely cures patients with pelvic wall recurrences following radical cystectomy.156 Some could perhaps be cured if given adjuvant radiotherapy.
The morbidity of postoperative irradiation is high because of the large volumes of small bowel that occupy the pelvis after cystectomy if the urologist does not use pelvic reconstruction techniques to displace small bowel loops from the tumor bed (e.g., omental pedicle flap or mesh). Two series reported an incidence of greater than 30% of small bowel obstruction when 50 Gy was delivered using conventional fractionation.157 If irradiation is envisaged as an accompaniment to cystectomy for advanced disease, it appears to be safer if delivered preoperatively. If this was not done, then the radiation volumes should be kept small, limited to the floor of the pelvis, and with a dose of no more than 45 Gy.
Salvage Radiation Therapy
Attempts to salvage patients with TCC of the bladder at the time of symptomatic or gross pelvic recurrence after radical cystectomy with radiation therapy (RT) or with combined chemotherapy and RT are usually unsuccessful. Some palliation can be achieved.156 In some centers, consideration is given to preoperative chemoradiation followed by attempted resection of the pelvic mass and intraoperative irradiation. There is no large published experience looking at this approach.
Bladder Preservation
Conservative Surgery
Partial Cystectomy
Surgical resection by partial cystectomy requires careful patient selection (i.e., the lesion is solitary and is located in a region of the bladder that allows for complete excision with a 2-cm tumor-free margin, such as the bladder dome). The portion of the bladder to be resected should be small enough to allow adequate bladder capacity. Contraindications include association with CIS in other sites of the bladder, prostatic urethral involvement, prior recurrent bladder or upper tract tumors, and bladder neck or trigone tumors in which ureteral reimplantation would be required to achieve an adequate margin. Given these constraints, only 6% to 19% of patients with primary, muscle-invading bladder cancer are potential candidates.158 Even for this select group of patients, local recurrence rates range from 38% to 78%; half of the recurrences appear in the first year and two thirds by 2 years.
Transurethral Resection of Bladder Tumor (TURBT)
Clinical complete response rates (assessed cystoscopically with repeat biopsy 3 weeks after initial TURBT) after TURBT for T2 and T3 cancers overall are in the 10% to 20% range.159,160 Five-year OS in 85 patients with grades 1 and 2 T2 cancers was an unacceptable 27%.161 Some studies have reported 10-year DSS as high as 76%,162 but these results included only patients who had no residual disease or no invasive disease on repeat biopsy and urine cytologic testing. Although potentially effective in a small proportion of favorable T2 tumors, TURBT is usually not sufficient as monotherapy in muscle-invading bladder cancer.
Radical External Beam Irradiation
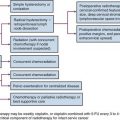
The total radiation dose used in modern series (Table 52-2) varied from 55 to 65 Gy, with 1.8 Gy to 2 Gy per fraction in North America, and from 50 to 55 Gy at 2.5 to 2.75 Gy per fraction in the United Kingdom.163–169,170,171–176 Most patients were treated with one fraction per day five times a week. Patient response was evaluated by cystoscopic examination and biopsy 3 to 6 months after completion of RT. Patients with residual tumor and no known metastatic disease underwent salvage surgery (range of salvage cystectomy rate, 13% to 24%) if they were suitable surgical candidates. Those who were unfit received palliative measures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree