Bladder Cancer
EPIDEMIOLOGY AND RISK FACTORS
More than 350,000 new cases of bladder cancer are diagnosed per year worldwide. In the United States, bladder cancer is the 4th most common cancer, with 72,570 new cases in 2013 (1). Bladder cancer is three times more common in men (54,610 cases) than women (17,960 cases). There will be an estimated 15,210 deaths from bladder cancer in 2013. The median age at diagnosis is 65 years.
The best established risk factor for bladder cancer development is tobacco use, which is linked to 50% of bladder cancer cases (2). Carcinogens from tobacco are filtered from the blood into urine, and have sustained contact with urothelial cells lining the bladder, ureter, and renal pelvis. Tobacco-related cancer risk is dose dependent and diminishes with tobacco cessation. Pelvic irradiation and exposure to the chemotherapeutic cyclophosphamide also increase the risk of developing bladder cancer. In some areas of the world, chronic bladder inflammation caused by schistosomiasis is linked to a squamous cell subtype of bladder cancer.
CLINICAL PRESENTATION AND DIAGNOSIS
Hematuria is the most common presenting symptom for bladder cancer. Hematuria may be gross or microscopic and is often painless, unless associated with clots and obstruction. Evaluation of asymptomatic patients for microscopic hematuria has not been an effective screening test for detection of bladder cancer.
When bladder cancer is suspected, workup should include urine cytology, cystoscopy, and an upper tract imaging study. Urine cytology has a sensitivity of 40%–60% with a specificity of greater than 90%. Spiral computed tomography (CT) can evaluate the upper urothelial tracts (renal pelvis and ureter), renal parenchyma, and lymph nodes. The definitive diagnosis can only be established by biopsy via transurethral resection of the bladder tumor (TURBT). Fluorescence in situ hybridization (FISH) of specific genes cannot be used to diagnose bladder cancer in the absence of a tissue biopsy, and the role of FISH in management of bladder cancer patients is not well established.
Transitional cell (urothelial) carcinoma is the most common subtype of bladder cancer (>90%), and can arise in other organs lined by transitional epithelium, including renal pelvis, ureter, and the proximal two-thirds of the urethra. Other histologic subtypes include squamous cell carcinoma (3% of bladder tumors in the United States), adenocarcinoma (2%; includes tumors at the bladder dome originating from the urachal remnant), and small cell tumors (1%). Further classification of transitional cell carcinoma subtypes includes the particularly aggressive micropapillary and sarcomatoid histologies. The remainder of this chapter will discuss management of transitional cell carcinoma.
STAGING
Urinary bladder cancer can be grouped into three general categories by stage at presentation: non-muscle-invasive (formerly described as superficial), muscle-invasive, and metastatic. Each differs in clinical behavior, primary management, and outcome.
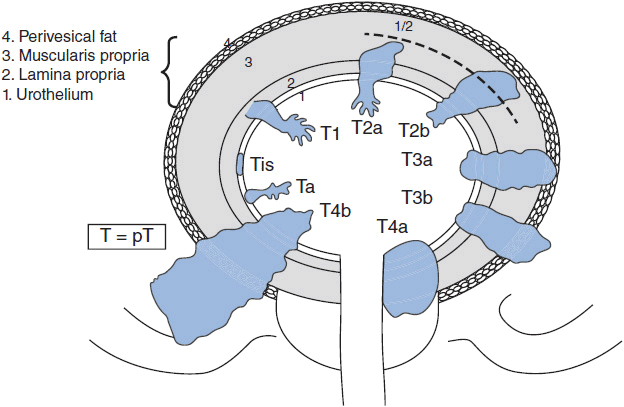
The primary bladder tumor is staged according to the depth of invasion into the bladder wall or beyond (see Figure 42-1). During TURBT, the visible tumor is resected along with sampling of involved or adjacent muscularis propria, to assess for the presence of muscle invasion. Non-muscle-invasive tumors include papillary mucosal tumors (Ta), flat mucosal tumors (carcinoma in situ [CIS], or Tis), and tumors involving the lamina propria (T1). Tumors that invade the muscularis propria layer comprise T2 disease. Tumors that penetrate beyond the muscle layer to the serosa are classified as T3. Tumors that involve contiguous pelvic structures such as the prostate, vagina, uterus, or pelvic/abdominal walls are classified as stage T4.
Patients with muscle-invasive bladder cancer require further staging workup beyond the initial abdominopelvic CT scan, including chest CT, complete blood count, blood chemistries, and consideration of a bone scan. Although the presence of urinary bladder cancer is associated with upper tract disease in <5% of cases, careful evaluation of the upper tracts is warranted due to the “field defect” from carcinogen exposure throughout the urothelium.
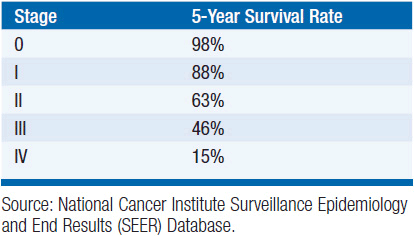
In the absence of lymph node or distant metastasis, T stage determines anatomic stage. Stage 0 disease constitutes Ta and Tis tumors. Stage I and II cancers have T1 and T2 tumors, respectively. Stage III cancers include T3 tumors and T4a tumors (involvement of prostate, uterus, or vagina). Stage IV cancers include T4b tumors (invading pelvic or abdominal wall), and tumors of any T stage that include lymph node or distant metastasis. Survival by stage is described in Table 42-1.
MANAGEMENT
 NON-MUSCLE-INVASIVE BLADDER CANCER (TA, TIS, T1)
NON-MUSCLE-INVASIVE BLADDER CANCER (TA, TIS, T1)
Non-muscle-invasive tumors constitute 70% of new diagnoses. Approximately 15%–20% of these patients will eventually progress to stage T2 or worse disease. Up to 70% of patients diagnosed with non-muscle-invasive cancer will have a local recurrence following initial therapy. Low-grade tumors and low-stage (Ta) disease tend to have a lower recurrence rate at about 50%, with a 5% rate of progression to higher stage disease. However, high-risk disease (high-grade disease, multifocal disease, or T1 disease) has a 70% recurrence rate and a 30% progression rate to stage T2+ disease. Due to high recurrence and progression rates, these patients require close urologic follow-up indefinitely, initially consisting of cystoscopy and urine cytology every 3 months. Fewer than 5% of patients with non-muscle-invasive bladder cancer will develop metastatic disease without exhibiting evidence of muscle invasion during the course of surveillance, underscoring the importance of close follow-up.
Patients at high risk for development of progressive or recurrent disease are generally considered candidates for adjuvant intravesical therapy. In general, this group includes patients with T1 tumors, multifocal CIS, high-grade tumors, or rapidly recurrent disease following initial resection. Standard options for intravesical therapy include immunologic agents such as Bacillus Calmette-Guérin (BCG) with or without interferon, or chemotherapy agents such as mitomycin C or doxorubicin. The goal of therapy is to decrease the rate of recurrence and progression.
Radical cystectomy of non-muscle-invasive bladder cancer is occasionally considered for patients with frequent recurrences, multiple tumors despite intravesical therapy, or intolerance of intravesical therapy.
 MUSCLE-INVASIVE BLADDER CANCER (T2-4)
MUSCLE-INVASIVE BLADDER CANCER (T2-4)
Surgical Approaches
The standard of care for muscle-invasive bladder cancer is radical cystectomy with bilateral pelvic lymph node dissection. In female patients, an anterior exenteration is performed, which includes removal of the bladder and urethra (the urethra may be spared if uninvolved and an orthotopic bladder reconstruction is to be performed), the ventral vaginal wall, and the uterus. In male patients, when the prostate stroma is involved with cancer or when there is concomitant CIS of the urethra, a cystoprostatourethrectomy is the treatment of choice.
Urinary diversion techniques include formation of an ileal conduit or construction of an orthotopic neobladder, an internal urinary reservoir that can drain via the urethra or abdominal wall. The morbidity of cystectomy is significant, with one case series at a high-volume center describing 64% of cases with one or more complications, 26% requiring readmission to the hospital, and 2.7% 90-day mortality (4).
The probability of survival from bladder cancer following cystectomy is determined by the pathologic stage of the disease. Five-year overall survival (OS) is markedly influenced by the presence of lymph node involvement. In contemporary series, survival for pathologic T2 disease ranges from 59% to 72%, whereas T3/T4 disease survival ranges from 26% to 58%. Node positive disease survival rates range from 26% to 35% (5–7).
Selective Bladder-Preserving Approaches
An alternative to radical cystectomy with lymph node dissection is organ preservation with trimodality therapy in selected patients. This approach requires the use of early cystectomy at the first sign of failure of local control. Successful approaches have evolved over the past three decades following the initial reports of the effectiveness of cisplatin against transitional cell carcinoma. Trials in the United States and Europe have evaluated various schedules of concurrent chemoradiotherapy with or without neoadjuvant or adjuvant chemotherapy, with iterative improvements in the treatment regimens concurrent with improved radiation techniques. Radiosensitizing drugs studied in these series, either singly or in various combinations, include cisplatin, carboplatin, paclitaxel, 5-fluorouracil, mitomycin C, and gemcitabine.
A phase III trial of 360 patients evaluated radiation therapy with or without 5FU and mitomycin C chemotherapy (8). Subjects largely had clinical T2-T4a disease. Subjects were also randomized to either whole-bladder or modified-volume radiation therapy. The primary endpoint of locoregional disease-free survival favored chemoradiotherapy at 2 years (67% vs. 54%; p = 0.03). Five-year OS also favored chemoradiotherapy, but the difference was not statistically significant (48% vs. 35%; p = 0.16).
The long-term outcomes of the approach at Massachusetts General Hospital via the Radiation Therapy Oncology Group (RTOG) was updated (9). Successive protocols involving 348 patients with clinical T2-T4a disease were combined. In summary, 72% of patients had complete response (CR) to induction chemoradiotherapy. Five-year OS was 52%. For clinical stage T2 disease (54% of subjects), the 5-year OS rate was 61%, as opposed to 41% for patients with T3-T4 disease. Greater than 70% of subjects retained their native bladder. Predictors of outcome include clinical T stage and CR to induction therapy.
The University of Erlangen has published the largest bladder-sparing study to date, involving 415 subjects that included 89 subjects with “high-risk T1” disease (10). This group included 126 patients who received radiation without any chemotherapy. The CR rate of all 415 patients was 72%. The 10-year disease-specific survival was 42%, and more than 80% of these survivors preserved their bladders.
The currently open North American protocol for bladder-sparing treatment (RTOG 0712) is a randomized phase II study comparing induction chemoradiotherapy with cisplatin plus 5FU with twice-daily radiation treatment, versus gemcitabine with once-daily radiation treatment. Consolidation chemoradiotherapy and adjuvant chemotherapy are given to subjects whose post-induction evaluation reveals <T1 disease; if ≥T1 disease is found, subjects proceed to prompt radical cystectomy followed by adjuvant chemotherapy.
Bladder preserving trimodality therapy should be one of the approaches considered in the treatment of selected patients with muscle-invasive bladder cancer. For example, eligible patients should have transitional cell carcinoma histology, absence of hydronephrosis, and tumors that can be maximally resected at TURBT. Published reports indicate comparable survival rates with contemporary cystectomy series (Table 42-2). This approach requires closely coordinated care among urologic surgeons, radiation oncologists, and medical oncologists. Patient-reported quality of life is favorable (11).
TABLE 42-2 FIVE-YEAR OVERALL SURVIVAL RATES ACROSS CONTEMPORARY STUDIES EVALUATING RADICAL CYSTECTOMY AND BLADDER PRESERVATION STRATEGIES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree