The urinary bladder, when empty, lies within the true pelvis.
The triangular superior surface of the bladder is covered with peritoneum; in females the body of the uterus usually overhangs this surface.
The apex of the bladder is directed toward the pubic symphysis and is joined to the umbilicus by the urachal remnant.
The posterior surface, the base of the bladder, faces downward and backward; in females, it is closely related to the anterior wall of the vagina. In males, the upper part of the bladder base is separated from the rectum by the rectovesical pouch; the lower part is separated from the rectum by the seminal vesicles and the deferent duct.
As the bladder fills, it becomes rounded or ovoid, and it lies directly against the anterior abdominal wall without any intervening peritoneum.
The ureters pierce the wall of the bladder base obliquely; the orifices of the ureters and the urethral orifice define the bladder trigone, the sides of which are approximately 2.5-cm long in the contracted state and up to 5.0 cm when distended.
In males, the bladder neck rests on the prostate; in females, it is related to the pelvic fascia surrounding the upper urethra (19).
Approximately 75% to 85% of new bladder cancers are superficial (Tis, Ta, or T1) (30).
Approximately 15% to 25% of new bladder cancers have evidence of muscle invasion at the time of diagnosis; it also develops in a number of other patients with superficial disease if the tumor recurs after conservative therapy. Of all patients with muscle-invasive bladder cancer, approximately 60% have evidence of muscle invasion at the time of initial diagnosis; 40% initially present with more superficial disease that later progresses (19).
The most common sites of tumor development are the trigone, lateral and posterior walls, and bladder neck.
Bladder cancer spreads by direct extension into or through the wall of the bladder. In some cases, the tumor spreads submucosally under intact, normal-appearing mucosa.
Papillary tumors tend to remain superficial; solid lesions are generally deeply invasive.
Perineural invasion and lymphatic or blood vessel invasion are common after tumor has invaded muscle.
The multifocal nature of bladder cancer probably accounts for many recurrences after transurethral resection (TUR) (17).
Lymphatic drainage is by the external and internal iliac and presacral lymph nodes.
The most common sites of distant metastases are lung, bone, and liver.
Between 75% and 80% of patients with bladder cancer have gross, painless, total (throughout urination) hematuria, although this may also be intermittent.
Approximately 25% of patients complain of vesical irritability; 20% have no specific symptoms, and their cancers are detected because of microscopic hematuria, pyuria, and so forth.
More than 95% of patients with biopsy-proven carcinoma in situ have positive urine cytology results.
Simultaneous presentations of bladder carcinoma and adenocarcinoma of the prostate are not rare (19).
Patients with bladder cancer should have a complete clinical history, physical examination (including careful rectal/bimanual examination), chest roentgenogram, urinalysis, complete blood cell count, liver function tests, complete cystoscopic evaluation, and bimanual examination under anesthesia both before and after endoscopic surgery (biopsy or TUR).
An intravenous urogram should be obtained before cystoscopy so that the upper tracts can be evaluated by retrograde pyelogram, cytology, brush biopsy, or ureteroscopy at the time of cystoscopy, if indicated. The number, size, and configuration of all tumors should be recorded and diagrammed.
Cystograms provide minimal information.
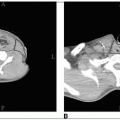
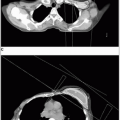
Computed tomography (CT) is widely used to help detect bladder wall thickening, extravesical extension, and lymph node metastases and is useful in follow-up. After TUR of a bladder tumor, CT findings that suggest extravesical extension may be caused by hemorrhage and edema; therefore, the results must be interpreted with caution.
Magnetic resonance imaging (MRI) in coronal or sagittal projections is sometimes useful in defining tumor extent.
Bone scans are obtained for patients with T3 or T4 disease and those with bone pain.
PET scanning, in patients with deep muscle invasion, may be useful in the detection of lymph node or distant metastasis.
The diagnostic workup is summarized in Table 31-1.
Two clinical staging systems, the Marshall modification of the Jewett-Strong system and the tumor-node-metastasis staging system of the American Joint Committee on Cancer (1), are widely used in the United States and abroad (Table 31-2). A shortcoming of the Marshall system is its failure to divide stage 0 into papillary and nonpapillary morphologies.
Both systems combine histologic findings from TUR specimens and clinical findings from bimanual examination under anesthesia.
Pathologic staging is based on histologic findings from cystectomy specimens. It is generally not possible for the pathologist examining TUR specimens to determine if tumor is confined to superficial muscle layers or has invaded the deep muscle.
The presence of muscle invasion implies that the lesion is stage B1, B2, C, D1, or D2 (T2-4b).
Although bimanual examination under anesthesia and radiography are helpful in further separating the various stages, under staging is common (19).
TABLE 31-1 Diagnostic Workup for Carcinoma of the Bladder | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||
In the United States, approximately 92% of bladder cancers are transitional cell carcinomas, 6% to 7% are squamous cell carcinomas, and 1% to 2% are adenocarcinomas. Small-cell carcinoma may occur (9, 16).
Squamous and/or glandular differentiation is seen in 20% to 30% of transitional cell carcinomas; the biologic behavior of these tumors does not differ significantly from that of pure transitional tumors (8).
Morphologically, bladder cancers can be separated into papillary, papillary infiltrating, solid infiltrating, and nonpapillary, noninfiltrating or carcinoma in situ.
At diagnosis, 70% are papillary, 25% show papillary or solid infiltration, and 3% to 5% are carcinoma in situ.
Sarcomas, pheochromocytomas, lymphomas, and carcinoid tumors account for most of the remaining 2%; because of their rarity, they are not discussed here.
Depth of tumor invasion (stage) and grade are important prognostic factors.
Presence of blood vessel or lymphatic vessel invasion is significant, even in the absence of positive lymph nodes and even if the tumor is confined to the lamina propria (7).
Carcinoma in situ, solid tumor morphology, large tumor size, multiplicity of tumors, muscle invasion, histologically positive lymph nodes, and obstructive uropathy are indicators of a poor prognosis (24).
TABLE 31-2 Comparison of Marshall and American Joint Committee on Cancer (1) Staging Systems for Bladder Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||