Polyomavirus (PyV) infections were first described in mice in 1952 as a cause of tumors in newborn animals. Since then, PyVs have been found in virtually all vertebrates, including primates, monkeys, cows, rabbits, birds, and fish. BK polyomavirus (BKPyV) was isolated in 1971 from a kidney transplant patient shedding decoy cells in the urine. The isolation of JC polyomavirus (JCPyV) was also reported in 1971 from postmortem tissues of patients with progressive multifocal leukoencephalopathy (PML), but PyV particles had been noted by electron microscopy in PML tissue sections as early as 1965. Since then, more than 10 human polyomaviruses (HPyVs) have been characterized molecularly, and there is serologic evidence that healthy adults are concurrently infected with at least six to seven different HPyVs. Clinical and histopathologic evidence of disease is currently available for six HPyVs ( Table 23.1 ), which almost exclusively affect immunocompromised patients. , This chapter reviews the recent literature on HPyVs in immunocompromised children undergoing cancer/chemotherapy, solid organ transplantation (SOT), or hematopoietic cell transplantation (HCT).
| Common Name | Genus | Taxonomic Name | Organ of Latency | Human Disease |
|---|---|---|---|---|
| BK polyomavirus | Betapolyomavirus | Human polyomavirus 1 | Kidney and urinary tract | Nephropathy, hemorrhagic cystitis |
| JC polyomavirus | Betapolyomavirus | Human polyomavirus 2 | Kidney, brain, blood cells | Progressive multifocal leukoencephalopathy |
| Merkel cell polyomavirus | Alphapolyomavirus | Human polyomavirus 5 | Skin | Merkel cell carcinoma |
| Trichodysplasia spinulosa | Alphapolyomavirus | Human polyomavirus 8 | Skin | Trichodysplasia spinulosa |
| New Jersey polyomavirus | Alphapolyomavirus | Human polyomavirus 13 | Unknown | 1 pancreatic transplant patient with muscle, skin, and eye disease |
| Human polyomavirus 7 | Deltapolyomavirus | Human polyomavirus 7 | Skin | Pruritic hyperproliferative keratinopathy in lung transplant patients |
Viral structure and life cycle
PyVs share a common morphology of non-enveloped icosahedral virion particles of 40 nm to 45 nm that contain a double-stranded DNA genome of approximately 5000 base pairs wrapped around histones. PyV genomes can be divided into three regions called the non-coding control region ( NCCR ), early viral gene region ( EVGR ), and late viral gene region ( LVGR ). After virion uptake and delivery of the viral genome to the nucleus, the NCCR coordinates, together with host cell factors, the start of the PyV replication cycle by initiating expression of the EVGR-encoded regulatory large T-antigen (LTag) and small-antigen (sTag). LTag and sTag inactivate tumor suppressor proteins pRB and p53 and shift cells into G 1 /S phase to provide necessary building blocks and recruit the host cell DNA polymerase complex for efficient bidirectional replication of the viral DNA genome from the NCCR ori. This is followed by NCCR -driven expression of the LVGR -encoded capsid proteins Vp1, Vp2, Vp3, and the regulatory agnoprotein. The LVGR also encodes viral micro-ribonucleic acids (miRNAs). Vp1 forms the outer shell of the virions consisting of 72 pentamers and assembles spontaneously in virus-like particles used for serologic studies. Vp2 and Vp3 are minor capsid proteins inside the particles adjacent to the viral DNA genome. Enlarged nuclei with prominent intranuclear inclusions consisting of densely packed PyV particles are the hallmark of PyV replication in the late phase of the viral life cycle. Immunohistochemistry for LTag using cross-reactive monoclonal antibody raised originally against the monkey SV40 LTag protein is commonly used for proven BKPyV or JCPyV pathology erroneously called “SV40 positive,” but detection of Vp1 or in situ hybridization has also been used by some centers. Although the principal virology of PyVs is conserved, there are significant differences which permit concurrent infections and mediate differences in host cell tropism, virus biology, and pathology. Thus the outer part of the Vp1 virions is more diverse, and is responsible for primary host cell tropism as judged from selective binding to gangliosides carrying differently branched sugar residues. These Vp1 domains are also the target of neutralizing antibodies. Conversely, the inner parts seem more conserved and mediate interactions between Vp1 pentamers or to Vp2. Binding of immunoglobulin G (IgG) are more frequently cross-reactive between different HPyVs and have less frequently neutralizing activity. The NCCRs of HPyVs differ in length, type, and number of transcription factor binding sites, and are critical for the secondary host cell tropism, which is realized inside the host cell nucleus by the exact timing of EVGR and LVGR expression. PyV micro-RNAs 5p and 3p target the EVGR transcripts and downregulate natural killer cell targets on the cell surface, thereby facilitating immune escape and latency. Taken together, HPyV biology subverts and hijacks the host cell metabolism without offering classic antiviral targets of high selectivity.
Epidemiology and risk factors
General population
The exact mode of natural BKPyV and JCPyV transmission is undefined but most likely involves contact with mucosal surfaces in the oral/pharyngeal, gastrointestinal, or respiratory tract. Seroprevalence studies indicate that primary infection with BKPyV occurs in toddlers, reaching IgG positivity rates of greater than 90% between the ages of 2 and 4 years. There are no known symptoms associated with primary BKPyV infection and a nonspecific, constitutional illness cannot be excluded. Primary JCPyV infection appears to occur significantly later as only 35% of adolescents have JCPyV-specific IgG antibodies compared with 60% of blood donors. In a study of 18 patients undergoing thymectomy in children owing to congenital heart surgery, the IgG seroprevalence of BKPyV and JCPyV was 70% and 25%, respectively. However, some differences in seroprevalence rates across different studies reflect patient age and waning antibody responses over time, but particular attention should be paid to the different antigens used (e.g., Vp1 monomers or fusion proteins, Vp1-pentamers or Vp1-virus-like particles).
HPyVs have been detected in the urine of 30% of healthy children and adults as well as 40% of stool samples from hospitalized children. Detection of BKPyV and JCPyV in human sewage systems supports the possibility of secondary indirect environmental exposures other than the direct transmission route (e.g., from child to child). Indeed, PyV particles are fairly resistant to environmental inactivation and can withstand heating to 60°C for 30 minutes and many disinfectants. Other routes of transmission are less well defined and even controversial (e.g., via transfusion, transplacental, seminal fluids, or organ transplantation), with the notable exception of kidney transplantation, where transmission has been shown to occur from donor to recipient.
After primary infection, BKPyV and JCPyV reach the renourinary tract presumably by DNAemia, where they preferentially infect the renal tubular and bladder epithelial cells in the case of BKPyV and the urothelial cells of the renal pelvis and the bladder in the case of JCPyV. , Moreover, detection of JCPyV DNA has been reported in tonsils, bone marrow, and the central nervous system. Taken together, large parts of the general human population have been infected with HPyVs, including BKPyV and JCPyV, but neither primary infection, persistence, nor shedding has been linked to significant pathology or disease in immunocompetent persons. Conversely, as outlined in Table 23.2 , immunocompromise appears to be a conditio sine qua non for HPyV disease, but the differences in incidence rates in the respective clinical settings strongly suggest that specific risk signatures beyond mere immunodeficiency appear to play a role. Thus BKPyV-associated nephropathy is most frequently diagnosed in adult and pediatric kidney transplant recipients at rates of 1% to 15%, but only rarely in non-kidney SOT or allogeneic HCT, despite similar or higher intensity of immunosuppression as evidenced by other opportunistic infectious diseases caused by Pneumocystis jirovecii or cytomegalovirus (CMV) replication. Conversely, hemorrhagic cystitis is most frequently seen after allogeneic HCT, but only rarely after kidney transplantation, wherein local toxic damage of the bladder urothelia from conditioning and impaired immune control are followed by increased inflammatory responses postengraftment. JCPyV-mediated PML has reached the highest rates in human immunodeficiency virus/AIDS before the availability of combination antiretroviral therapy or in refractory relapsing multiple sclerosis treated with natalizumab, but less data are available for treatment with dimethyl fumarate or fingolimod, or in SOT or HCT. Accordingly, risk-adapted consultation, screening, and intervention are currently recommended for these respective patients.
| Pediatric Population | Clinical Manifestation (Reported Pediatric Rates) |
|---|---|
| BKPyV | |
| Kidney transplant | Nephropathy (5%-15%) Hemorrhagic cystitis (rare) |
| Hematopoietic cell transplant | Hemorrhagic cystitis (8%-25%) Nephropathy (rare) |
| Liver Transplant | Nephropathy (rare) |
| Heart transplant | Nephropathy (rare) |
| Lung transplant | Hemorrhagic cystitis (rare) |
| Malignancy | Hemorrhagic cystitis (rare) |
| JCPyV | |
| HIV/AIDS, refractory multiple sclerosis | Progressive multifocal leukoencephalopathy |
| Kidney transplant | Nephropathy (rare) Progressive multifocal leukoencephalopathy (very rare) |
| Malignancy | Progressive multifocal leukoencephalopathy |
| Less common clinical manifestations: gastrointestinal, pulmonary, ophthalmologic, hepatic, neurologic, cancer | |
a Nephropathy and hemorrhagic cystitis associated with BKPyV are the predominant clinical manifestations affecting kidney transplant and hematopoietic cell transplant recipients, respectively. Progressive multifocal leukoencephalopathy associated with JCPyV is the predominant clinical manifestation affecting patients with human immunodeficiency virus/AIDS and refractory multiple sclerosis.
Kidney transplantation
The key steps of BKPyV reactivation to nephropathy have been described in detail, but they were mostly derived from adult patients after kidney transplantation. These include the following:
- 1.
Low-level viruria in approximately 5% to 10% of patients with residual urine production before kidney transplantation
- 2.
High-level replication with urine BKPyV loads greater than 10 million copies/mL and decoy cell shedding in 20% to 50% of patients after transplantation
- 3.
Detection of BKPyV DNA in plasma in 10% to 40% of patients after transplantation
- 4.
Histologically proven BKPyV-associated nephropathy with little inflammation and baseline allograft function (PyVAN-A)
- 5.
Increasing allograft damage due to BKPyV replication and inflammation decreasing kidney allograft function (PyVAN-B1, -B2, -B3); and
- 6.
Irreversible fibrosis and tubular atrophy causing decline in allograft function (PyVAN-C).
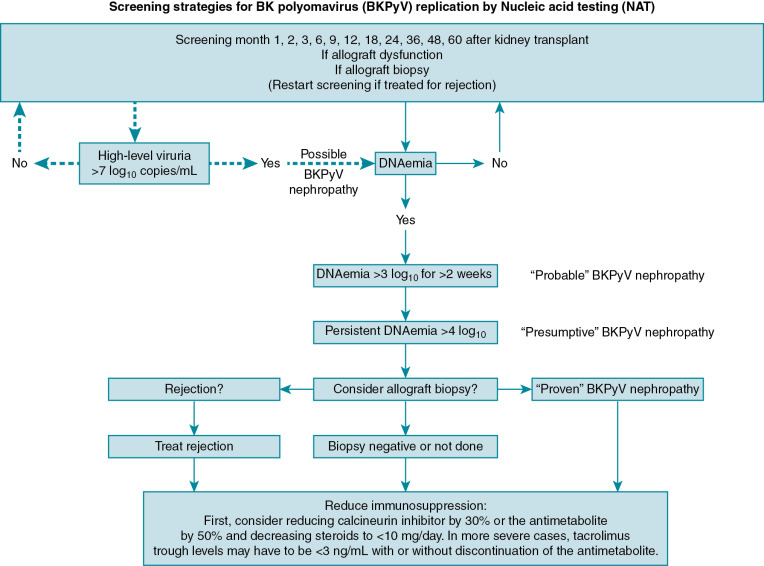
Accordingly, screening for high-level viruria and/or DNAemia using nucleic acid testing (NAT) is recommended to identify patients with persistent DNAemia who would benefit from preemptive reduction in immunosuppression.
Most of the literature describing BKPyV infection replication and disease in children has been published in the past decade. In a prospective clinical and laboratory study from 2002 to 2005 in Italy, Ginevri and colleagues followed up with 62 children who received basiliximab and standard maintenance triple therapy with a calcineurin inhibitor, mycophenolate mofetil, and corticosteroids. Only 3 of the 62 patients received induction that included antithymocyte globulin. Blood and urine samples were collected at months 1, 3, 6, 9, 12, 18, 24, 36, and 48 after transplant for NAT testing (458 samples, average of 7 samples per patient after transplant). The cumulative risk of viruria was 64% (95% confidence interval 53% to 78%) and that of DNAemia was 22% (95% confidence interval 13% to 35%), and was first detected at a median of 3 months (range 1 to 24 months for viruria and 1 to 18 months for DNAemia) after transplant. Using a protocol to reduce immunosuppression, no cases of BKPyV-associated nephropathy occurred in this series.
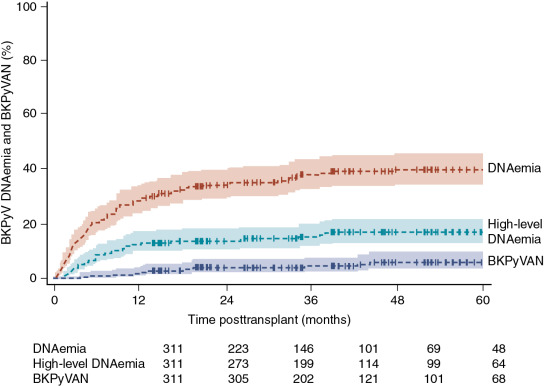
Risk factors for BKPyV replication after kidney transplant have included the depth and type of immunosuppression, including the use of antithymocyte globulin for induction or rejection treatment and the level of exposure to tacrolimus and mycophenolate mofetil. Other reported risk factors include human leukocyte antigen (HLA) mismatches, deceased donation, older age, male gender, donor antibody high/recipient antibody low, and ureteral stents. In their analysis of 313 children receiving a kidney transplant in Europe, Höcker and colleagues reported the cumulative incidence of DNAemia and biopsy-proven nephropathy after kidney transplant ( Fig. 23.1 ). In a multivariate model, they found that a higher degree of immunosuppression (odds ratio [OR] 1.3, P < .01), tacrolimus use (vs. cyclosporine) (OR 3.6 P < .01), younger recipient age (OR 1.1 per year, P < .001), and obstructive uropathy (OR 12.4, P < .01) were associated with higher risk of BKPyV infection.
The serostatus of the donor and recipient may also contribute to posttransplant BKPyV risk. Ali and colleagues conducted a retrospective analysis of pediatric kidney transplant patients at their center in Canada from 1986 to 2007. All patients had follow-up for at least 1 year after transplant. Using an enzyme-linked immunosorbent assay to detect IgG against the BKPyV Vp1 virus-like particle, the authors tested stored blood samples from donors and recipients. An antibody titer of 1:2560 or less was categorized as a low titer and a titer of 1:10,240 or more was defined as a high titer. Of the 94 included transplant recipients, 34% had low anti-BKPyV IgG serostatus and 66% had high serostatus before transplant. Consistent with published reports for healthy children, pretransplant antibody titers were higher with increasing age, especially after the age of 6 years. Blood was also available to test 40 donors, 73% of whom had antibody titers in the high anti-BKPyV IgG serostatus group. Recipients with a low serostatus had a significantly higher risk of BKPyV DNAemia in the first year after transplant. The highest risk of BKPyV DNAemia was found in children with high donor serostatus and low recipient serostatus. Although BKPyV-specific IgG mediate part of the antiviral immune response, a higher antibody status may be a surrogate of recent exposure or reactivation, and hence reflect a higher tissue BKPyV load in the allograft that cannot be countered by sufficient immune effector functions in the recipient with low BKPyV-specific immunity evidenced by low antibody titers. Although the numbers were small, BKPyV DNAemia occurred in 4 of 7 (57%) transplants from a high donor serostatus to a low serostatus recipient, in none of the 3 transplants in which both the donor and the recipient had low serostatus, and in only 1 of 26 (4%) transplants in which the recipient had high serostatus before transplant. Unlike CMV and Epstein-Barr virus (EBV), it is not standard to test for donor and recipient antibodies against BKPyV before kidney transplant in children or adults. This may change, however, as more research becomes available on the functional and surrogate role of antibody titers and specificities in donor-recipient pairs, and may help to better determine the posttransplant risk of uncontrolled BKPyV replication and disease.
This notion may be extended to differences in BKPyV subtype antibody titers. BKPyV can be divided into four major subtypes (I, II, III, and IV) and each subtype can be further divided in subgroups yielding a total of 12 different categories of BKPyV strains. BKPyV subtype I is found in patients worldwide, whereas subtype IV is found in patients mostly from East Asia and Europe. Subtypes II and III are rarely reported. Subtypes II, III, and IV and subgroups Ib1 and Ib2 are also known to be distinct serotypes. Thus a subtype mismatch arising from transplanting of a donor graft harboring BKPyV subtype IV into a recipient with antibody titers to Ib may be followed by preferential replication of the donor BKPyV subtype. Momynaliev and colleagues examined BKPyV subtypes among 6 pediatric kidney transplant recipients and 10 adult controls at their center in Russia from 2008 to 2009. They found that 66% of the identified BKPyV isolates were Ib2 and 24% were IVc2. More research is needed to determine the role of BKPyV genotypes and serotypes in children with respect to posttransplant high-level viruria, DNAemia, and disease, and the risk-benefit of BKPyV mitigation and graft survival through informed screening and organ allocation.
Nonrenal solid organ transplantation
Few studies have examined the risk of BKPyV replication in children after nonrenal SOT with most of the literature limited to cross-sectional analyses of patients who were several years after transplant, when the risk of BKPyV replication events is presumably lower. In a study of 59 pediatric liver transplant recipients in Israel by Amir and colleagues, blood and urine samples were tested at a single time point at least 1 month after transplant and retested if they were positive. At a median of 5 years after liver transplant, 9 of 59 (15.3%) had viruria, and 1 of 59 patients (1.7%) had low-level DNAemia. In all 9 of the patients with viruria, BKPyV replication was transient and there was no longer evidence of DNAemia or viruria by their next clinic visit 5 months later. In Germany, Brinkert and colleagues tested 100 pediatric liver transplant recipients for BKPyV and JCPyV in urine, and plasma was tested only if the initial urine result was positive above 100,000 copies/mL. Of the 100 included patients in this cross-sectional analysis, 15 (15%) had isolated BKPyV viruria at a median of 6 years after transplant, but no DNAemia was identified.
In contrast to liver transplantation, recipients of thoracic transplants (heart and lung) typically receive higher doses of immunosuppression to prevent rejection, placing them at greater risk for infectious disease events. Ducharme-Smith and colleagues conducted a cross-sectional analysis of pediatric heart transplant recipients in the United States by collecting urine samples at regular clinic appointments. Since 2006, urine testing was performed only in patients with a history of chronic kidney disease and all patients were screened starting in 2012. Blood testing for BKPyV DNA was performed only if urine testing was positive. Of the 83 patients screened for viruria at a median of 3.3 years after transplant, 28 (34%) had viruria. Of these 28 patients, 7 had test results for DNAemia (representing 8% DNAemia among the total study population). In multivariate analysis, patients in whom BKPyV viruria developed were significantly more likely to have evidence of EBV detection in the blood. In a follow-up study, the authors prospectively collected urine and blood samples from 10 consecutive pediatric heart transplant recipients from 2013 to 2015. Samples were collected before transplant and at 1 week and months 3, 6, 9, 12, and 15 after transplant. Quantitative blood and urine NAT was only performed if the result of the initial qualitative urine NAT was positive. The 10 subjects had follow up for 15 months after transplant, during which time viruria developed in 2 of 10 and 1 of 10 (10%) had DNAemia.
Several reviews have summarized the literature describing cases in adults and children with BKPyV replication after heart or lung transplant. , In adults after heart transplant, studies have reported a viruria risk of 19% and a DNAemia risk of 5%. After lung transplant, 33% of recipients have been found to have viruria, with less data available on the risk of DNAemia. No studies have systematically examined the risk of BKPyV replication after pediatric lung transplant or in children with cancer. As summarized in later text, there are mainly case reports of BKPyV nephropathy in children after nonrenal SOT, especially in those receiving heart or lung transplants. However, the exact risk of nephropathy in the native kidneys of children with SOT remains unknown.
Hematopoietic cell transplantation
After HCT, BKPyV is typically associated with hemorrhagic cystitis, leading to significant morbidity and possibly an increased risk of death. Less commonly, BKPyV nephropathy was diagnosed in the native kidneys of HCT recipients, and presented similar to what is seen in patients after kidney transplantation. Risk factors for BKPyV replication after HCT include the type of graft, HLA mismatch, recipient CMV serostatus, and conditioning with antithymocyte globulin. In a time-varying analysis including 88 children undergoing allogeneic HCT, graft-versus-host disease (GVHD) was not associated with the subsequent development of hemorrhagic cystitis.
In the most comprehensive study to date assessing BKPyV replication in children after HCT, Cesaro and colleagues prospectively examined patients younger than 18 years after allogeneic HCT at their center in Italy from 2005 to 2012. Plasma and urine samples were collected for BKPyV NAT at baseline, weekly until day 30, then at days 45, 60, 90, and 100 after transplant. Of the 107 patients enrolled, 20 patients (18.7%) had hemorrhagic cystitis, 90% of whom had BKPyV viruria and 80% of whom had BKPyV DNAemia. Among the 87 patients in whom hemorrhagic cystitis did not occur, 64% had viruria and 47% had DNAemia after transplant. Gaziev and colleagues assessed children younger than 17 years undergoing allogeneic HCT for sickle cell anemia or thalassemia at their center in Italy from 2004 to 2009. They enrolled a total of 117 patients over the study period, including 64 of whom were monitored prospectively for BKPyV DNA in the blood and urine weekly until day 100 after transplant. Of the 64 patients monitored prospectively, 60 (94%) had at least one sample positive for viruria and 52 (81%) had at least two samples positive for viruria. Regarding DNAemia, 34 (53%) had at least one positive sample and 18 (28%) had at least two samples positive for DNAemia. These studies support findings that BKPyV viruria, DNAemia, and hemorrhagic cystitis are common in children undergoing HCT. No studies have systematically evaluated the epidemiology of hemorrhagic cystitis in children with cancer who have not undergone HCT.
Clinical manifestations
Clinical disease associated with HPyV in children is almost exclusively limited to immunocompromised patients (see Table 23.2 ). In fact, one of the first cases of BKPyV-associated nephropathy presenting as interstitial nephritis occurred in a child with hyper-IgM syndrome (CD40 ligand/CD40 deficiency). BKPyV is most commonly associated with direct kidney injury (nephropathy) after kidney transplant or hemorrhagic cystitis after allogeneic HCT. However, in HCT recipients, nephropathy of their native kidneys can also develop, even in the absence of hemorrhagic cystitis, but there are no reliable data about the incidence. Conversely, it is undefined why hemorrhagic cystitis rarely develops in kidney transplant patients, non-kidney SOT recipients, and children undergoing treatment for cancer. It is possible that a urotoxic insult damaging the urothelial cells may cause rarefication of the mucosal cell layer, on top of which high-level BKPyV replication promotes progression to hemorrhagic cystitis. Less commonly, PyV infections have been linked with gastrointestinal, pulmonary, ophthalmologic, hepatic, and neurologic disease. JCPyV has been much less associated with clinical manifestations in SOT, HCT, or children with cancer, among which the diagnosis of PML is the most devastating one. Of interest, only rare cases of JCPyV nephropathy have been seen in adult and pediatric kidney transplantation at rates of less than 1% to 5%, respectively. , , This is notable because the rate of serologic mismatch between donor and recipient, and hence specific immune effector mismatch, would be predicted to occur in approximately 20% among adults and 50% in children assuming average seroprevalence rates of 60% and 35%, respectively. Thus as yet undefined factors must be involved in mitigating JCPyV-mediated pathology in the kidney as opposed to the brain, despite the high homology and similarity between JCPyV and BKPyV. Both viruses have been associated with malignant transformation and cancer, including the demonstration of chromosomal integration and NCCR alteration in BKPyV variants detected in urothelial carcinoma in kidney transplant patients. ,
Nephropathy
Proven BKPyV nephropathy is diagnosed in about 5% of pediatric renal transplants and is associated with chronic graft damage, premature decline in function, and an at least 10% attributable risk of graft loss. Most cases of BKPyV nephropathy occur in the first year after kidney transplant. Graft outcomes appear to be worse in patients with a later diagnosis of nephropathy and in those who did not respond to preemptive reductions in immunosuppression.
BKPyV is also recognized as a cause of nephropathy in the native kidneys of children who have received an HCT, mostly published as case reports. Verghese and colleagues described two children in whom biopsy-proven BKPyV nephropathy developed after HCT. A 10-year-old was found to have a serum creatinine level of 1.5 mg/dL 3 years after cord blood transplant for chronic myelogenous leukemia. Severe GVHD was treated with intense combination immunosuppression, including corticosteroids, mycophenolate mofetil, tacrolimus, infliximab, and photopheresis. Without signs of hemorrhagic cystitis, the serum creatinine eventually rose to 3 mg/dL, at which point BKPyV viruria and DNAemia were detected. Five years after transplant, the patient had chronic kidney disease with a serum creatinine level of 1.7 mg/dL. The second patient was a 13-year-old presenting with acute kidney injury, hypertension, and edema 16 months after allogeneic HCT for Fanconi anemia. At that time the serum creatinine level was 1 mg/dL and the patient was being treated with amphotericin for fungal disease and immunosuppression for GVHD. The serum creatinine peaked level at 2.3 mg/dL 2 years after HCT in conjunction with BKPyV viruria and DNAemia. The patient’s kidney function worsened and died after declining dialysis.
BKPyV has also been reported in the native kidneys of children after nonrenal SOT, primarily in heart transplant recipients. Lorica and colleagues reviewed six children in whom BKPyV nephropathy developed after heart transplant including a 14-year-old presenting with posttransplant lymphoproliferative disorder 12 years after heart transplant. The serum creatinine concentration rose to 3 mg/dL and the patient had a positive test result for BKPyV DNAemia and viruria with a kidney biopsy demonstrating nephropathy and positive immunohistochemistry for the SV40 LTag. The patient started dialysis but died. In their cross-sectional study of 83 pediatric heart transplants, Ducharme-Smith and colleagues identified 1 patient in whom biopsy-proven BKPyV nephropathy developed and who required a kidney transplant for subsequent end-stage kidney disease. They also noted that patients with BKPyV viruria had lower estimated glomerular filtration rates compared with those heart transplant recipients without viruria. So far, there are no reported cases of BKPyV nephropathy after pediatric liver transplant. In their cross-sectional study of 59 pediatric liver transplant recipients, Amir and colleagues found no difference in renal function between recipients with or without viruria, similar to the findings reported by Brinkert and colleagues in their cohort of 100 pediatric liver transplant recipients.
Hemorrhagic cystitis
Hemorrhagic cystitis associated with BKPyV infection is most commonly reported in children undergoing allogeneic HCT. Hemorrhagic cystitis can develop early after transplant (<1 week), typically related to conditioning chemotherapy, and cyclophosphamide in particular. Late-onset hemorrhagic cystitis (>1 week) is more often secondary to infections. Although it is believed that BKPyV contributes to most cases of late-onset hemorrhagic cystitis after HCT, it is important to note that the exact mechanism for BKPyV-associated hemorrhagic cystitis remains unknown. It is also unclear why hemorrhagic cystitis is largely limited to the allogeneic HCT population, whereas kidney transplant recipients, who have similarly high urine BKPyV loads, rarely have hemorrhagic cystitis. Many have hypothesized that cystitis occurs from some combination of residual urothelial damage to the bladder from conditioning chemotherapy, BKPyV replication from primary or reactivation infection in the face of immunosuppression, and inflammation from engraftment after transplant. BKPyV-associated hemorrhagic cystitis is much less common in children with cancer who have not received an HCT, primarily reported as cases. In one of the larger series, Cheerva and colleagues described 14 nontransplant pediatric oncology patients treated with high-dose cyclophosphamide or ifosfamide, in whom cystitis developed in 4 (29%) despite hyperhydration and mesna prophylaxis. Three of the four patients with cystitis had positive test results for BKPyV viruria and hematuria persisted for 10 to 16 weeks.
The all-cause incidence of hemorrhagic cystitis after HCT is reported to be about 25% and is associated with morbidity from prolonged inpatient lengths of stay and severe urinary discomfort. Early hemorrhagic cystitis is typically associated with conditioning chemotherapy, whereas later-onset cystitis (>1 week after transplant) can be associated with other causes, including viral and bacterial infection. In its most severe form, hemorrhagic cystitis can lead to life-threatening bleeding complications requiring aggressive surgical interventions. Reported risk factors for late-onset hemorrhagic cystitis after HCT include high-level BKPyV viruria (>7 log 10 ), myeloablative conditioning, unrelated mismatched donors, cord blood transplant, peripheral blood stem cells, cyclophosphamide, busulfan, antithymocyte globulin, total body radiation, CMV, human herpesvirus 6 (HHV-6) infection, and older age (>7 years). , ,
The prospective analysis by Cesaro and colleagues has provided the strongest evidence for the association between BKPyV replication and hemorrhagic cystitis in children after HCT. In addition to collecting plasma and urine samples during the first 100 days after transplant, routine urinalyses to screen for hematuria were performed daily while patients were hospitalized and weekly after discharge until day 100. Hemorrhagic cystitis was defined as gross hematuria plus clinical signs of cystitis. Of the 107 patients enrolled, cystitis developed in 20 (18.7%) at a median of 25 days after HCT (range 7 to 98 days). The duration of gross hematuria was a median of 13 days (range 2 to 71 days). About half of the cases of cystitis occurred before platelet or neutrophil engraftment. The authors examined how viruria and DNAemia predicted cystitis in the first 30 days after transplant. Viruria greater than 7 log 10 had a positive predictive value of 14% and a negative predictive value of 98% for later cystitis. DNAemia greater than 1000 copies/mL performed slightly better, with a positive predictive value of 39% and a negative predictive value of 100% for later cystitis. In a multivariate model, BKPyV DNAemia greater than 1000 copies/mL predicted hemorrhagic cystitis with an adjusted hazard ratio (HR) of 6.1 (2.2 to 17.1, P < .001). After a median 2.5 years of follow-up, hemorrhagic cystitis was associated with higher risk of overall mortality (HR 2.6, 1.2 to 5.8, P < .02).
Other studies in children have supported that BKPyV DNAemia can predict subsequent hemorrhagic cystitis after HCT. Laskin and colleagues analyzed samples from a previously enrolled prospective cohort of 88 allogeneic HCT transplant recipients in Cincinnati from 2010 to 2011. Cystitis was identified by chart review and subjects also had routine urine analyses weekly while hospitalized. Hemorrhagic cystitis was defined as gross hematuria. BKPyV DNAemia results obtained on clinical request were combined with an analysis of stored samples obtained days 0 to 14, days 15 to 85, and day 100 after transplant. Of the 88 subjects, hemorraghic cystitis developed in 17 (19%) at a median of day 25 (interquartile range 18 to 42 days) after transplant. There was no difference in the maximum grade of acute GVHD, platelet engraftment, neutrophil engraftment, or absolute lymphocyte counts between those with and without hemorrhagic cystitis. A time-varying analysis showed that peak DNAemia (1 to 9999 copies/mL) had an HR of 5.3 (2 to 14.6, P < .01) and more than 100,000 copies/mL had an HR of 34.3 (4.6 to 256.1, P < .01) for later cystitis. HHV-6 DNAemia and older age were also independently associated with hemorrhagic cystitis.
Disease prophylaxis/prevention
There are currently no agents that have demonstrated efficacy in preventing BKPyV replication. Knoll and colleagues conducted a double-blind, placebo-controlled, randomized trial of 3 months of levofloxacin starting 5 days after kidney transplant in 154 adults. Levofloxacin did not decrease the risk of BKPyV viruria or DNAemia, but it did increase the risk of quinolone-resistant bacterial infections and possibly tendinitis. Intravenous immunoglobulin preparations contain high concentrations of anti-BKPyV antibodies. Although some have hypothesized that immunoglobulin formulations could prevent BKPyV infection, no trial data are available to support its use. Despite the fact that many patients receive immunoglobulin infusions for hypogammaglobulinemia after HCT, the risk of BKPyV infection and cystitis remains high. In the future, development of a BKPyV vaccine may allow recipients to be immunized before transplant to prevent infection. ,
Pretransplant approaches to prevent infection
More research is needed to determine optimal strategies for assessing prior to transplantation the posttransplant risk of BKPyV replication and disease. Before transplant, it is standard to test donors and recipients for EBV and CMV antibody status, but there is not enough evidence to support similar testing for BKPyV antibodies. Children who are seronegative for BKPyV may have a higher risk of developing DNAemia and nephropathy after transplant, but replication and disease has been shown to develop in seropositive patients. As mentioned earlier, in a retrospective analysis of pediatric kidney transplant recipients, Ali and colleagues found that recipients with a low serostatus had a significantly higher risk of BKPyV DNAemia in the first year after transplant, and the highest risk of BKPyV DNAemia was found in children with low antibody titers who received transplants from donors with high antibody titers. Finally, Koskenvuo and colleagues reported that among six children in whom hemorrhagic cystitis developed after allogeneic HCT, five were seronegative for BKPyV IgG and IgM before transplant. Of note, the response to intravenous and intravesical cidofovir coincided with mounting a serologic response, raising the possibility that previous cidofovir-attributable effects were actually confounded by emerging BKPyV-specific antibody and cellular immune responses.
Posttransplant screening to prevent infection
Awaiting the development of an effective prophylactic medication or vaccine against BKPyV, prevention of viral disease currently relies on screening for BKPyV viruria and/or DNAemia after transplant to permit timely reduction of immunosuppression, whenever possible. Ginevri and colleagues demonstrated that a stepwise protocol to lower immunosuppression in response to asymptomatic DNAemia prevented the development of any cases of BKPyV nephropathy, without corresponding increase in rejection, in 62 children after kidney transplant. Specifically, in response to increasing BKPyV DNAemia, a patient’s calcineurin inhibitor was first reduced by 15% to 20%. If DNAemia persisted, the mycophenolate mofetil was then decreased by half and then discontinued. The optimal screening protocol for BKPyV replication after kidney transplant remains unknown. Pape and colleagues conducted a survey among 90 pediatric nephrologists in Europe and found 26% of providers performed screening for viruria alone, 37% screened both urine and blood, and another 37% screened only for DNAemia. Most physicians (47%) screened patients at months 1, 2, 3, 6, 9, and 12 after transplant.
In support of screening for viruria first, urine samples may be easier to obtain and a negative test result has a very high negative predictive value for BKPyV DNAemia and nephropathy. On average, viruria usually precedes DNAemia by 4 weeks and DNAemia precedes nephropathy by 8 weeks. In favor of screening for DNAemia first, the specificity of BKPyV viruria for nephropathy is low and pediatric transplant patients are frequently already having blood sampling. Most centers have determined their own screening protocols depending on the laboratory resources and expertise available. A screening algorithm for children after kidney transplant is shown in Fig. 23.2 . Because most cases of BKPyV nephropathy occur in the first 2 years after kidney transplant, it is reasonable to screen more frequently early after kidney transplant or after treatment for rejection. There is no current evidence or guidelines to suggest screening for BKPyV after nonrenal transplant. However, this may change if more research demonstrates that BKPyV DNAemia can predict hemorrhagic cystitis after allogeneic HCT or if the risk of BKPyV replication and disease after nonrenal SOT is comparable to that seen after kidney transplant.