74.1
Introduction and history
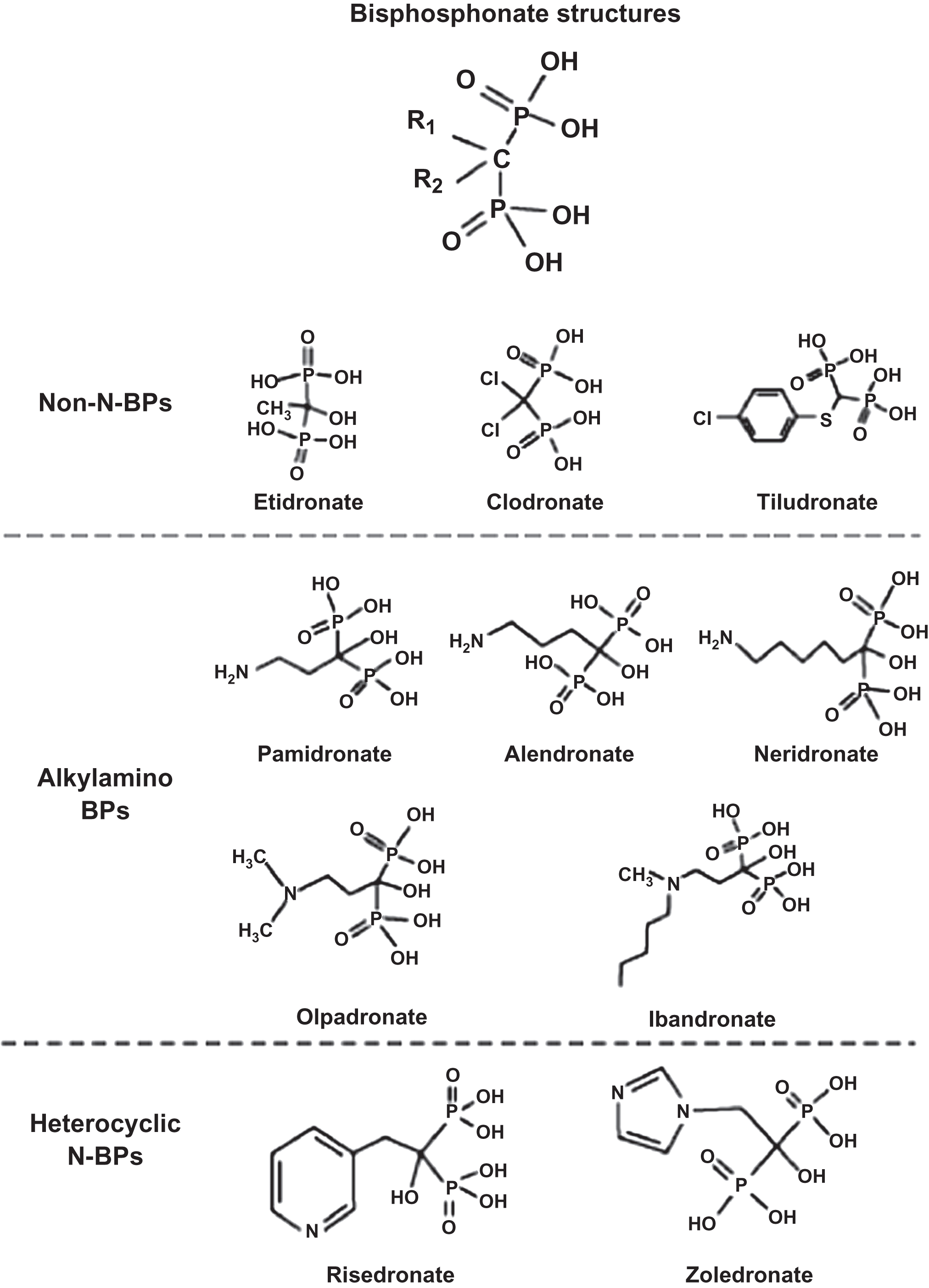
Bisphosphonates are analogs of naturally occurring compounds, pyrophosphates (P–O–P). P–O–P are byproducts of adenosine triphosphate (ATP) metabolism but have little or no biological activity in healthy individuals because they undergo rapid enzymatic degradation by ubiquitous pyrophosphatases (e.g., acid phosphatase and alkaline phosphatase) (the exception is hypophosphatasia, a condition leading to buildup of pyrophosphates due to lack of alkaline phosphatase). The substitution of a carbon atom in place of oxygen makes the molecule a bisphosphonate (P–C–P) ( Fig. 74.1 ) . Bisphosphonates have a high affinity for bone and are not metabolized. Bisphosphonate that does not bind to bone is rapidly excreted in the urine unchanged . Renal excretion is accomplished by glomerular filtration as well as proximal tubular secretion, so that the clearance of bisphosphonates exceeds the clearance of inulin [an accurate measure of glomerular filtration rate (GFR)]. Because bisphosphonates are not metabolized, they also have no effect on the pharmacokinetics (PK) of other drugs. Thus modification of dosing of other medications (e.g., warfarin) is not required when a bisphosphonate is added.
Bisphosphonates are poorly absorbed when taken by mouth . This poor absorption is related to the heavy negative charge of the bisphosphonate molecule, which makes transport across lipophilic cell membranes difficult and facilitates complexing with divalent cations (e.g., calcium and magnesium). Absorption is significantly reduced or blocked if the drug is taken with food or in high concentrations of divalent cations (e.g., with calcium supplements or mineral water). Even when correct dosing instructions are followed, less than 1% of an oral dose is absorbed, but this small amount has a powerful effect on bone turnover. Yet, there are many clinical circumstances in which absorption may be doubtful: malabsorption conditions, including celiac disease, gastrojejunostomies, small bowel resection, and dumping syndromes, where rapid transit time through the intestinal tract may mitigate absorption. Management decisions might be simpler if bisphosphonate blood levels could be measured in clinical practice. However, surrogate markers must be used, namely, bone mineral density (BMD) and bone turnover markers (BTM), to gain a sense of whether the bisphosphonate is being absorbed and having a positive biological effect on bone. Surrogate markers are useful but imperfect indicators of bone biological effects of bisphosphonate action, although surrogates are valuable when properly employed and interpreted .
Bisphosphonates were initially developed as a spinoff from the study of polyphosphates to inhibit soap from binding to glass. The scientific observations of William Neuman, Herbert Fleisch, Graham Russell, and David Francis in the 1960s led to the knowledge that the first bisphosphonate studied (etidronate) inhibited bone resorption or inhibited mineralization in rat bones, depending on the dose . Because etidronate in higher doses inhibits tissue mineralization, the first clinical use of etidronate was in 1969 in a child with myositis ossificans progressiva . The positive clinical outcome that a bisphosphonate could benefit disease associated with metabolic bone derangements led to registration of bisphosphonate use in many diseases that have benefited humankind ( Table 74.1 ). There has been intense scientific and clinical refinement in understanding the mechanisms of action of bisphosphonates, as well as their similarities and their differences .
| Tracers for nuclear medicine bone scans |
| Inhibition of mineralization/calcification (e.g., heterotopic ossification and dental plaque) |
| Reducing bone resorption |
| Osteoporosis |
| Paget disease |
| Multiple myeloma |
| Bone metastases from solid tumors |
| Hypercalcemia of multiple etiologies |
| Miscellaneous disorders (e.g., osteogenesis imperfecta and fibrous dysplasia) |
74.2
Pharmacokinetics and pharmacodynamics
Bisphosphonates have a high affinity for bone, and, for practical purposes, bone is the only tissue that takes up bisphosphonates. This selective tissue uptake is due to two distinct properties: (1) affinity for the hydroxyapatite crystals that are exposed as a consequence of osteoclastic bone resorption (physiochemical effect) and (2) uptake by osteoclasts and inhibition of bone resorption (cellular effect) .
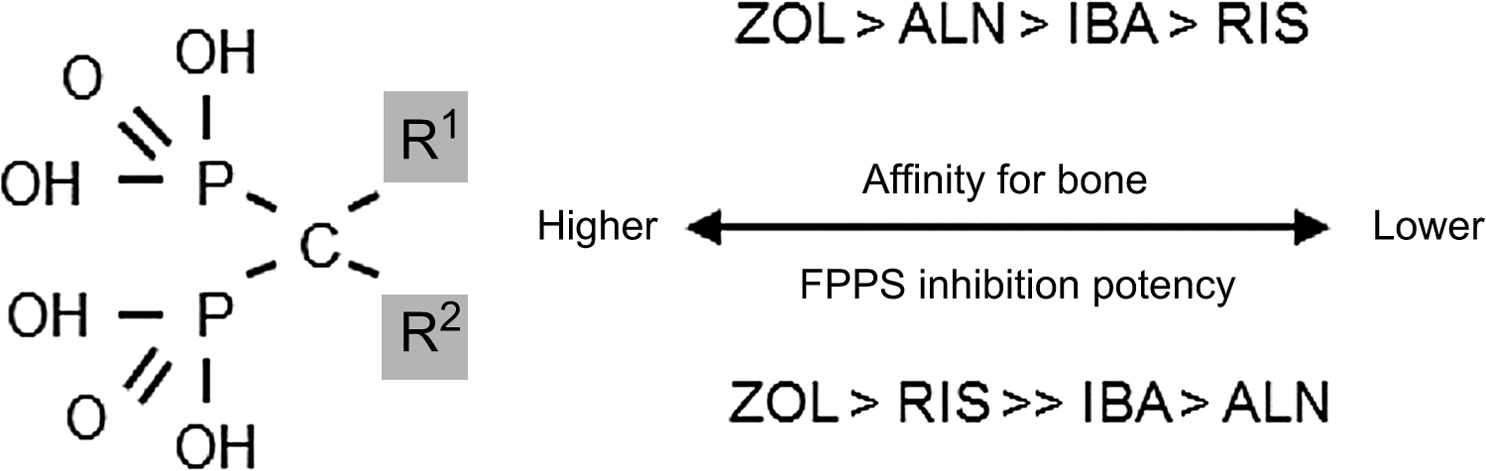
From the P–C–P bond common to all bisphosphonates, the differences in the chemical structure among the bisphosphonates are determined by their side-chain moieties . These side-chain differences explain, in part, differences between the aminobisphosphonates: their affinity and adherence to the hydroxyapatite surface, their diffusion into bone, their displacement from this adherence and physiochemical binding when bisphosphonates are discontinued (offset), and the differences in their potency to inhibit the mevalonic acid pathway enzyme, farnesyl pyrophosphate synthase (FPPS) ( Fig. 74.2 ) . Thus bisphosphonates differ from one another in vitro as well as in vivo. How these differences translate into clinically different outcomes will be explored later in this chapter.
With regard to mechanisms for reducing bone resorption, there are two different molecular structures to be considered: nonaminobisphosphonates (etidronate and clodronate), and aminobisphosphonates (alendronate, risedronate, ibandronate, pamidronate, and zoledronic acid). Nonaminobisphosphonates have similar physiochemical but different cellular effects from aminobisphosphonates. Nonaminobisphosphonates disrupt the ATP metabolic pathway in osteoclasts that leads to programmed osteoclast death (apoptosis), while aminobisphosphonates inhibit the enzyme FPPS. Inhibition of FPPS leads to reduced production of specific intracellular proteins necessary for osteoclast function and survival . The net result is reduced bone resorption by osteoclasts as well as more rapid osteoclast apoptosis. Studies have also demonstrated that bisphosphonates may have antiapoptotic effects on osteoblasts and osteocytes . Data suggest that bisphosphonates increase serum osteoprotegerin (OPG) levels (an osteoblast-derived protein that is a decoy receptor for RANK-ligand) and that these increases in OPG correlate with increases in BMD . It is unknown at this time if the increase in OPG reflects a direct effect of bisphosphonates on osteoblasts or an indirect effect, for example, by altering osteoclastogenesis and thereby altering the catabolism of OPG. Monkkonen et al. have shown that nitrogen-containing bisphosphonates induce the intracellular accumulation of an ATP analog ApppI [triphosphoric acid 1-adenosine-5-yl ester 3-(3-methylbut-3-enyl) ester], inhibiting a different enzyme in the mevalonic acid pathway, adenine nucleotide translocase, which also contributes to osteoclast apoptosis . Hence, bisphosphonates may affect not just one but two intracellular enzymes in the mevalonic acid pathway, leading to disruption of osteoclast cellular function.
In addition, attention has also been focused on potential differences in the cellular effects of FPPS activity or crystal affinity between the aminobisphosphonates that might also explain some of the differences in the onset and/or the duration of their action (e.g., the ability to see earlier effects on fracture risk reduction or to apply longer dosing intervals than the initially studied daily dosing of bisphosphonates in clinical trials) . There are clear differences in potency for FPPS inhibition: zoledronic acid and risedronate have a greater ability to inhibit FPPS activity than does ibandronate or alendronate . In addition, alendronate, ibandronate, and zoledronic acid have a greater affinity for hydroxyapatite than risedronate. Hence, it is difficult to understand the differences in the pharmacodynamics that might possibly translate into clinical differences between the most widely utilized aminobisphosphonates. It must be emphasized that there are no comparisons of the skeletal binding, skeletal half-lives, offset of activity, or FPPS activity among bisphosphonates in similar patient populations. There are short-term studies in humans comparing differences between urinary excretion of clodronate, alendronate, risedronate, and zoledronic acid, and short-term PK studies comparing terminal elimination half-lives between risedronate and alendronate that suggest PK differences . How these differences translate into potential differences in the clichéd term “skeletal half-life” among bisphosphonates is unknown. The only head-to-head clinical trial between two aminobisphosphonates done in the same randomized population, the weekly alendronate and risedronate trial (FACT, Fosamax-Actonel Comparator Trial), showed differences in BMD and BTM responses between these two effective agents . However, in FACT, there were no fracture data, nor any comparative PK or offset data to answer the fundamental clinical question: are there differences between bisphosphonates with respect to fracture risk reduction or in the offset of effect after discontinuation?
The concept of the “recycling” of bisphosphonates was introduced in 1999 in measuring urinary excretion of alendronate by high-pressure liquid chromatography after alendronate discontinuation . It was observed that upon alendronate discontinuation, the molecule was excreted in the urine unchanged. This observation and the work of Nancollas et al. raised the probability that the released bisphosphonate that reenters the systemic circulation during the bone remodeling process could still be metabolically active and, by reattaching to newly formed bone resorption cavities, maintain clinical activity . In addition, there are data demonstrating that bisphosphonates with lower bone affinity have increased penetration of the osteocyte canalicular network and tend to distribute into osteocytes . The amount and rate of the rerelease of bisphosphonates from and their reattachment to a new bone multicellular unit might differ among bisphosphonates according to their affinity for bone . The rerelease of bisphosphonates might not only result from detachment from bone surfaces but also from coming out of the osteoclasts through a transmembrane process termed “transcytosis” as suggested by data from Coxon and Rogers . Bisphosphonate “recycling” could explain maintenance of BMD and BTM reduction seen in long-term studies after several years of administration . These observations will be examined in greater detail later in this chapter.
74.3
Treatment of postmenopausal osteoporosis: efficacy
The pivotal trials that led to registration of alendronate, risedronate, ibandronate, and zoledronic acid for the treatment of postmenopausal osteoporosis (PMO) fulfilled the required primary end point required by the US Food and Drug Administration (FDA) for registration—significant reduction in incident vertebral fractures over 3 years compared with a placebo group using once daily dosing. The intermittent dosing formulations of the oral (weekly and monthly) and quarterly intravenous ibandronate were subsequently approved on the basis of noninferiority end points: that intermittent dosing induced a noninferior increase in spinal BMD as the fracture-proven daily dosing . Because there are no head-to-head studies with fracture as a prespecified end point, it is unknown whether one bisphosphonate is superior to another for reducing fractures.
The FDA-approved indications differ among the bisphosphonates for nonvertebral and hip fracture reduction, which were secondary end points in the pivotal vertebral fracture clinical trials. Alendronate gained an FDA registration for hip fracture but not for nonvertebral fracture risk reduction, while risedronate gained registration for nonvertebral but not for hip fracture reduction, and ibandronate for neither nonvertebral nor hip fracture reduction. The PMO registration trial for zoledronic acid had two primary end points, vertebral and hip fracture reduction, and these efficacy end points were achieved, as well as the secondary end point: nonvertebral fracture risk reduction.
The lack of an FDA indication does not mean that one bisphosphonate may not have an effect on reducing the incidence of a specific type of fracture, even though that bisphosphonate did not meet FDA product-labeling requirements. For example, in the Fosamax International Trial (a nonregistration study), significant reduction in nonvertebral fracture incidence was observed with alendronate . In addition, in the risedronate HIP (Hip Intervention Program) trial, there was a significant reduction in hip fracture incidence in the largest hip fracture-bisphosphonate trial completed to date . Yet, neither of these bisphosphonates has an FDA label for the specific indication (alendronate for nonvertebral and risedronate for hip fracture). In the ibandronate registration clinical trial, no effect was seen on nonvertebral or hip fracture events in prospective analysis .
FDA registration does not permit registration based on secondary end points if the primary end point has not been achieved; in addition, it does not permit registration on the basis of post hoc analyses. Hence, the lack of an FDA registration does not mean that a particular bisphosphonate does not have a beneficial effect on reducing fracture risk at a non-FDA product-labeling registered site. The only means to secure clinical differences between bisphosphonates are head-to-head fracture comparisons between bisphosphonates.
The clinical choice of a bisphosphonate, if chosen only by FDA labeling, would restrict clinician flexibility. In the current health-care economic climate, clinicians are faced with trying to manage patients who may not tolerate one bisphosphonate as opposed to another for unclear reasons. Health-care provider plans may have one “preferred” bisphosphonate over another, creating unnecessary scenarios in which both the doctor and the patient have hurdles to overcome to gain access to an alternative bisphosphonate that might be preferred by medical judgment, although this has become less of an issue with the availability of low-cost generics. Compliance with osteoporosis treatments is suboptimal, as it is with the treatment of many chronic diseases . In addition, there is evidence that patients may prefer a weekly or monthly bisphosphonate formulation over the fracture-proven daily dosing formulation . Better persistence and adherence to therapy may result in better outcomes (e.g., fracture risk reduction) for some patients than for those patients who are less compliant with their medication . In the real world of clinical practice, having choices is good.
Surrogate markers of fracture such as BMD increases and reduction in BTM are imperfect indicators of bone strength, since fracture risk reduction may be seen in clinical trial subjects whose bone density remains stable when compared with the placebo group who lose BMD . Data from the extension of the zoledronic acid PMO registration trial suggest a nearly linear relationship between the increase in total hip BMD and reduction in fracture risk . Furthermore, there was an independent robust contribution observed between the reduction in the resorption bone marker (C-telopeptide) and fracture risk reduction, an observation suggested several years ago from a metaanalysis examining this relationship . Certainly, while fracture risk reduction remains the most important clinical outcome, surrogate markers (both changes in BMD and BTM) are important measurements used in clinical practice to monitor bisphosphonate efficacy .
It is important to point out that there are data suggesting mechanisms, whereby bisphosphonates may increase bone strength independent of changes in bone mineral content or bone turnover . In an important study, Borah et al. showed that by preserving horizontal trabeculae in an oophorectomized minipig model, risedronate improved bone strength in part due to microarchitectural preservation . Cortical porosity increases in bone with aging and contributes to impairment in bone strength and is also associated with a higher risk of hip fracture . Data suggest that bisphosphonates reduce cortical porosity that may be another independent mechanism, whereby bisphosphonates increase bone strength beyond BMD or turnover . There also may be effects of bisphosphonates to increase bone strength by affecting bone size . Although bisphosphonates improve bone strength by increasing bone mineral content and reducing bone turnover, other factors may also contribute to improvements in bone strength that future research will better clarify.
74.4
Bisphosphonate efficacy beyond 3 years
Bisphosphonate registration for the treatment of PMO requires evidence of incident vertebral fracture risk reduction over a 3-year period as compared with placebo. What about effects on fracture risk reduction beyond 3 years of use? The bisphosphonate clinical trials all have extension data beyond the initial preplanned 3-year registration trial, yet none have maintained the initial randomized population sample size for which the power calculations for fracture risk reduction were performed . Hence, evidence for continued efficacy beyond 3 years is limited by dropouts and the selection bias that is fundamentally inherent in subset analysis. In addition, in the two extension trials that had a rerandomized population, the primary end point was changed to BMD rather than fracture risk reduction, which is one of the reasons the FDA discounted all data from any of the extension studies .
The alendronate and zoledronic acid registration clinical trials are the two trials that used a rerandomized withdrawal study design; hence, these two trials should be examined in detail, since they form the efficacy basis for the analyses that make recommendations concerning duration of bisphosphonate use .
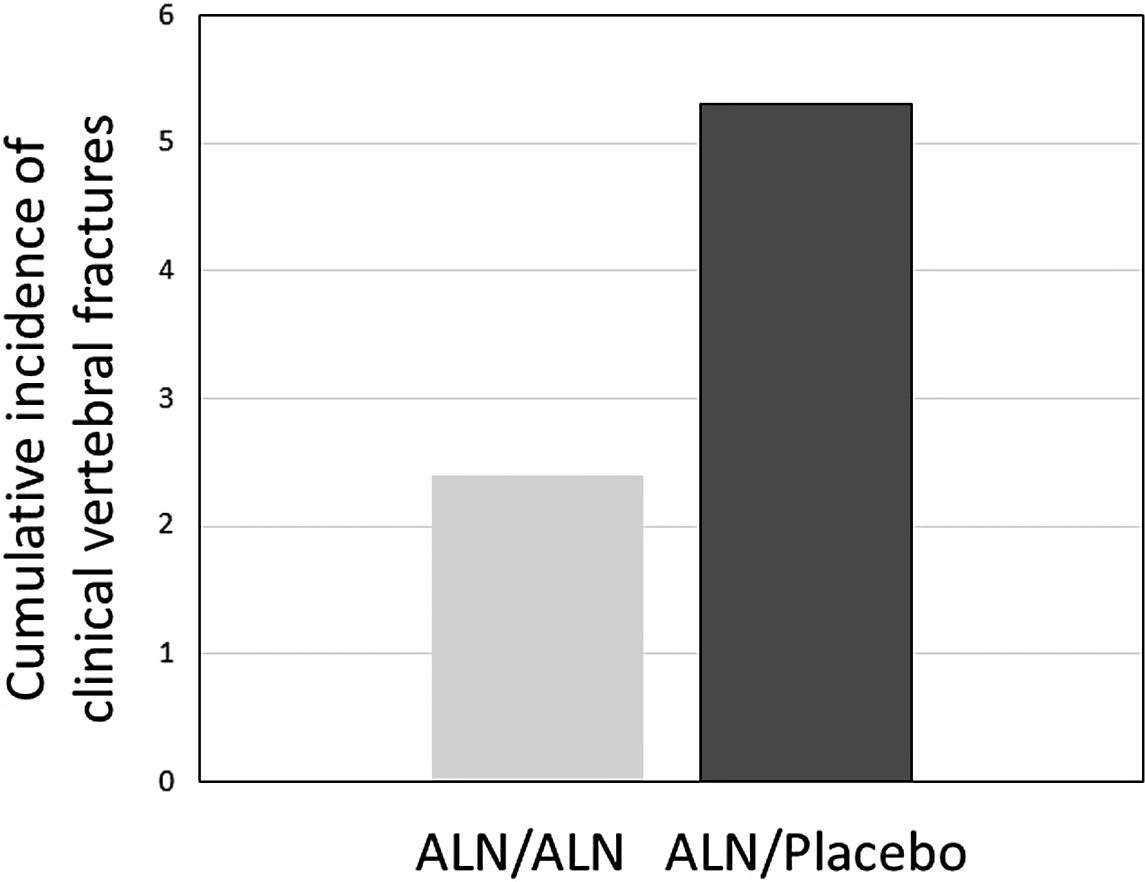
In the FLEX (Fracture Intervention Long-term Extension) trial, patients were observed for 10 years on alendronate (5 mg/day for 2 years and then 10 mg/day thereafter) versus treatment with alendronate for 5 years and then no alendronate for 5 years. Spine BMD increased more (+3.8%) and bone turnover reduction was maintained at a reduced level in the long-term treated groups as opposed to the placebo group. In the FLEX 5-year study, hip BMD declined a small but significant amount, and NTX increased 30% from baseline within 1 year of discontinuation but then plateaued so that by the end of the 5-year “off therapy” it was still 32% higher than the continuation group and below the pretreatment baseline value. There were no differences in morphometric or nonvertebral fracture events at 10 years between the two groups in FLEX. There were fewer clinical vertebral fractures in the long-term treated group (55%) that met statistical difference from the placebo group (about 2% vs 5%) ( Fig. 74.3 ) . These data suggest that there is an increase in clinical vertebral fracture risk after stopping 5 years of alendronate, and, that this greater risk was seen in the FLEX population with and without prevalent vertebral compression fractures and whose T-score entering FLEX was −2.0 or lower. In a post hoc analysis of the FLEX data, nonvertebral fracture risk reduction was also observed in the subset of patients without prior vertebral compression fractures but only in those with T-scores entering FLEX of −2.5 or lower at the femoral neck hip .
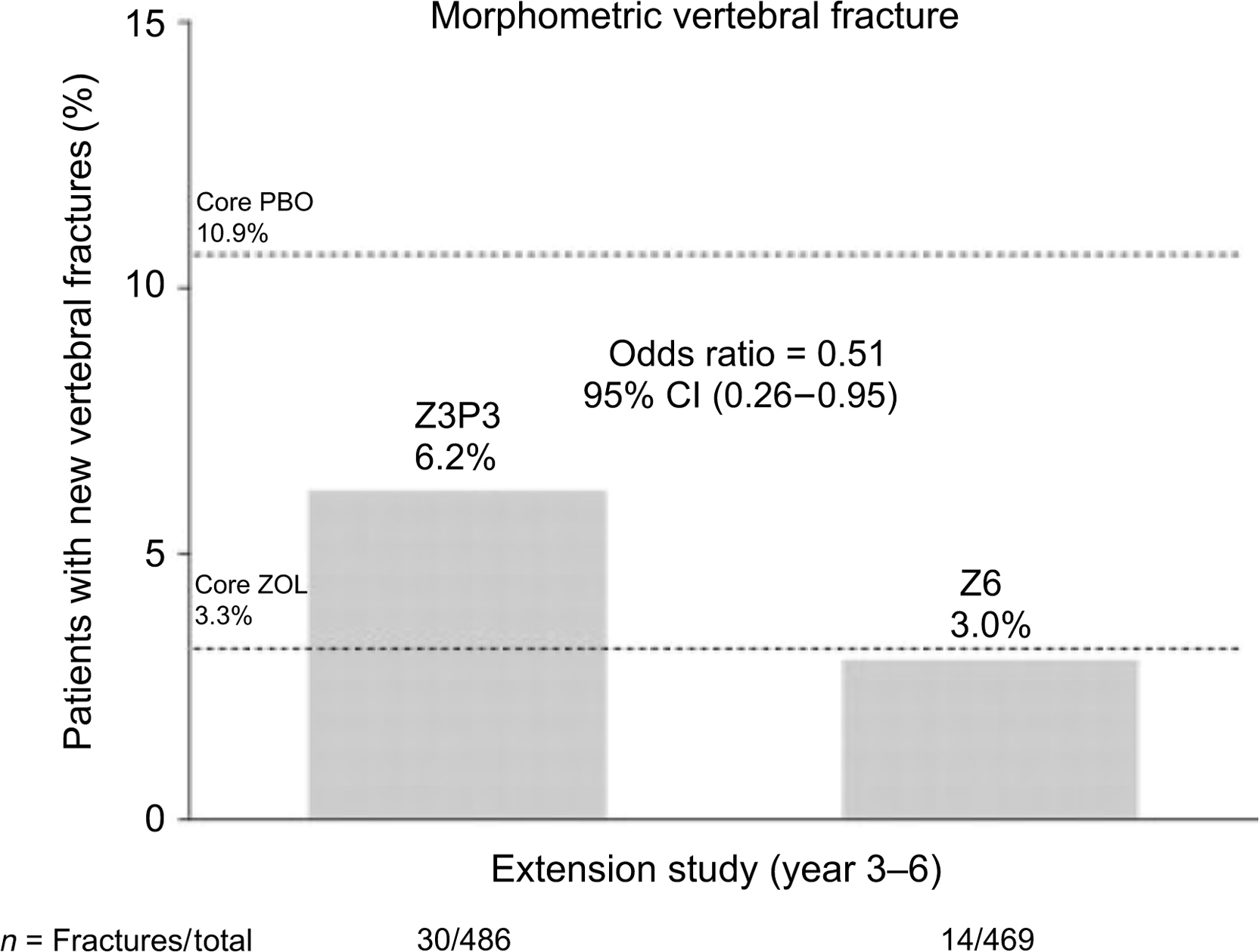
The zoledronic acid (HORIZON) extension data did not have a placebo group that continued off therapy from the original 3-year pivotal trial . This study had two extension trials. The first extension trial had two arms: the continuation [6 years of zoledronate (Z6)] administration and the discontinuation group that received the initial 3 years of active treatment then went to placebo (Z3P3). In the discontinuation group, there was a significantly greater risk of morphometric vertebral fractures (51%) compared with the group that continued 6 years of zoledronic acid ( Fig. 74.4 ). Since the majority of subjects in the HORIZON registration trial had prevalent vertebral fractures, the continuation efficacy is most likely confined to those higher risk patients with vertebral compression fractures . The numbers of morphometric vertebral compression fractures were too small to assess a specific T-score level where efficacy was maintained or lost. In the second extension of the HORIZON trial, postmenopausal women previously treated with zoledronic acid for 6 years were rerandomized to continue treatment or switch to placebo for an additional 3 years (“Z9” and “Z6P3” groups) . Three morphometric vertebral fractures were reported with 9 years of treatment compared with five reported with 6 years of treatment. Clinical fractures were similar between the two groups, reported in 10 of the patients who continued treatment for 9 years and in 9 patients who received 6 years of therapy, suggesting that there was no additional benefit in extending treatment with zoledronic acid therapy from 6 to 9 years.
Optimal dosing and patient selection for zoledronic acid remains an open question. In a 2-year study of patients with “osteopenia,” dosing once was almost as effective as yearly for preservation or gains in BMD . A 3-year open-label study of zoledronic acid showed that the antiresorptive activity of single doses of 1–5 mg seems to persist for at least 3 years in postmenopausal women with “osteopenia” . In another study of patients with “osteopenia,” reduction in fractures was shown with dosing every 18 months .
Finally, while head-to-head studies between bisphosphonates are still lacking, a recent clinical trial comparing the antifracture efficacy of risedronate with teriparatide, an anabolic agent, in patients with severe osteoporosis showed that the risk of new vertebral and clinical fractures was significantly lower in patients receiving teriparatide than in those receiving risedronate .
74.5
Bisphosphonate safety
Bisphosphonates are exceptionally safe medications. Treatment of millions of patients worldwide has been associated with side effects but no clear toxicity as pharmacologically defined with the exception of rare effects on renal function with intravenous bisphosphonates. The safety issues to be highlighted are gastrointestinal adverse effects, including esophageal cancer, musculoskeletal side effects, acute phase reaction, atrial fibrillation, renal safety, osteonecrosis of the jaw (ONJ), and atypical femur fractures.
74.6
Gastrointestinal adverse effects and esophageal cancer
Oral daily bisphosphonates have been associated with esophageal ulcers, esophagitis, and bleeding; however, these side effects lessened with the advent of weekly (alendronate, risedronate) or monthly (ibandronate, risedronate) preparations. Concern has emerged about an association between oral bisphosphonate use and an increased risk of esophageal cancer , which remains unvalidated. The bulk of evidence points away from an association between bisphosphonate and esophageal cancer , although a large nested case–control study of over 15,000 adults with gastrointestinal cancer compared with approximately 78,000 matched controls found a doubling of the risk of esophageal cancer among patients who had 10 or more prescriptions for bisphosphonates or who had taken these drugs over 3 years compared with patients not prescribed bisphosphonates (about 30% vs 17%) , it is possible that the difference is due to enhanced case-finding in bisphosphonate users. Further studies looking at the potential risk for carcinogenicity are clearly needed; nevertheless, the current data do not support a causal association between oral bisphosphonates and esophageal carcinoma.
74.7
Musculoskeletal side effects
Musculoskeletal pain did not show up as a common side effect in placebo-controlled trials but has been reported, postmarketing, with all bisphosphonates . The pain is usually diffuse and can range from a spectrum of mild and transient pain to severe and prolonged pain. There is no evidence that bisphosphonates induce rhabdomyolysis and serum creatine phosphokinase levels are not elevated by bisphosphonates. While this may be a class effect, some patients may tolerate one bisphosphonate better than another. The musculoskeletal side effect usually disappears within days after stopping the bisphosphonate.
74.8
Acute phase reaction
Approximately 30% of treatment-naïve patients receiving their first doses of intravenous bisphosphonate (or high-dose oral) experience an acute phase reaction (fever, headache, myalgia, arthralgia, and malaise) occurring within 24–36 hours and lasting up to 3 days . The incidence is reduced about 50% by acetaminophen (500–1000 mg before and for 24–48 hours post infusion, decreases with subsequent infusions and appears less common with higher 25-hydroxyvitamin D levels) .
74.9
Atrial fibrillation
In the 3-year HORIZON Pivotal Fracture Trial , subjects treated with zoledronic acid had an increased incidence of serious adverse events of atrial fibrillation (1.3% with zoledronic acid vs 0.5% with placebo, P <.001), but no imbalance in nonserious atrial fibrillation events. There were no significant differences in the rates of stroke, myocardial infarction, or deaths due to cardiovascular events nor was there any relation to the timing of drug infusion, acute phase reactions, calcium levels, or other electrolyte abnormalities. This report prompted additional investigation of the risk of atrial fibrillation in post hoc analyses of other bisphosphonate trials and reviews of health-care databases. None of these studies found an association between the use of bisphosphonates and atrial fibrillation. Zoledronic acid was not associated with an increased risk of atrial fibrillation in the HORIZON Recurrent Fracture Trial (subjects were older and presumably at higher risk) . Similarly, there was no increase in the rate of atrial fibrillation in the first extension of the HORIZON pivotal trial , although there was a small increase in cardiac arrhythmias (combined serious and nonserious) in the Z9 group in the second extension of the HORIZON trial . None of the oncology trials where subjects received zoledronic acid in doses that were approximately 10 times the dose for osteoporosis (i.e., 4 mg monthly instead of the dose for osteoporosis which is 5 mg yearly) showed an increase in the rate of atrial fibrillation. Similarly, post hoc analyses of studies with other bisphosphonates, including alendronate, risedronate, and ibandronate, did not show a statistically significant increase in the risk of atrial fibrillation . Population-based case–control studies are conflicting, some showing an increase in the risk of atrial fibrillation in women with past (but not current) use of alendronate, others showing no increased risk . A more recent nested case–control Italian multicenter study showed an almost twofold increased risk of atrial fibrillation in current bisphosphonate users compared with those who had stopped bisphosphonate treatment for more than 1 year . Besides the conflicting data associating bisphosphonates with atrial fibrillation, there is no clear biologically plausible mechanism by which this might occur. The FDA recommends that patients should not stop taking their bisphosphonate medication because of this theoretical concern, stating that “across all studies, no clear association between overall bisphosphonate exposure and the rate of serious or nonserious atrial fibrillation was observed.”
74.10
Renal safety
Rapid infusion of intravenous bisphosphonates may induce acute renal failure, especially in patients with reduced renal function or dehydration . Use of other agents that have nephrotoxic potential, such as nonsteroidal antiinflammatory drugs or diuretics, also increases the risk for renal dysfunction. To avoid compromise of renal function, bisphosphonates should not be given to patients with reduced GFR (<30 mL/min for risedronate and ibandronate, <35 mL/min for alendronate and zoledronic acid). Zoledronic acid should never be administered in less than 15 minutes and, if there are any concerns, a slower infusion time (30–60 minutes) seems to be an even safer approach. Though the FDA specifically recommends measuring creatinine clearance before each zoledronic acid infusion, estimated GFR (eGFR) calculation is also acceptable. In the phase 3 HORIZON study, a small but significant number of postmenopausal women treated with zoledronic acid demonstrated increases in serum creatinine concentration 9–11 days after the second infusion; the serum creatinine concentration returned to normal before the next infusion and there was no difference in eGFRs in drug- versus placebo-treated patients over the course of the trial . In the 3- and 6-year extension data (6 and 9 years of zoledronic acid therapy), there were no differences in eGFR between placebo and annual zoledronic acid . Intravenous ibandronate, dosed for osteoporosis (3 mg every 3 months), has shown no significant renal toxicity if treated patients have eGFRs≥30 mL/min and no baseline renal comorbidities .
Oral bisphosphonates are not nephrotoxic and, in fact, are effective to reduce fracture risk without any negative effect on renal function–based post hoc analyses in patients with an eGFR as low as 15 mL/min . The FDA warning not to use bisphosphonates in patients with an eGFR less than 30 or 35 mL/min is based more on the lack of data in this population. Approximately 50%–60% of administered bisphosphonate is excreted unchanged by the kidneys with the remainder taken up by bone; thus an oral dose in patients with lower GFR should be reduced.
Despite the lack of evidence regarding the use of bisphosphonates in patients with severe renal impairment and end-stage renal disease, treating patients suffering from fragility fractures with half the usual dose of bisphosphonates for up to 3 years should be considered, only after the diagnosis of osteoporosis is confirmed by a bone biopsy, since such patients may have fractures due to other forms of metabolic bone disease (renal osteodystrophy) .
74.10.1
Osteonecrosis of the jaw
ONJ (see also Chapter 81 : Lessons from bone histomorphometry on the mechanisms of action of osteoporosis drugs) is defined as exposed necrotic bone in the maxillofacial region, not healing after 8 weeks, in patients with no history of craniofacial radiation . It has been described in patients receiving chronic bisphosphonate therapy as well as subjects not using bisphosphonates. The incidence is estimated to be 0.7 per 100,000 patient-year exposure, although it is difficult to obtain accurate estimates because not all cases of ONJ are reported and not all cases reported are truly ONJ. Risk factors for developing ONJ include invasive dental procedures and preexisting dental disease, cancer and anticancer therapy, severe immunosuppression, intravenous bisphosphonates, duration of exposure to bisphosphonate therapy, glucocorticoids, and smoking. A causal link between bisphosphonate use and ONJ has not been established although it appears to be likely. Despite a number of potential mechanisms including oversuppression of bone turnover, the pathophysiology of ONJ remains poorly defined.
None of the clinical trials using bisphosphonates for treatment of osteoporosis or Paget disease, which included over 60,000 patient-years of exposure, identified ONJ prospectively . In the HORIZON trial with intravenous zoledronic acid for osteoporosis, a retrospective review identified one case of ONJ in the treatment group and another in the placebo group . In the first extension of the HORIZON trial, there was one case of ONJ in the Z6 group which resolved with appropriate treatment , and there were no cases of ONJ identified in the second extension of the HORIZON trial . In a study with romosozumab versus alendronate , with 4093 patients enrolled and followed for a median of 33 months, ONJ was reported in one patient receiving alendronate for more than 12 months and a second patient changed to alendronate after 1 year of romosozumab. A Task Force of the American Society for Bone and Mineral Research performed a comprehensive review and recommended that clinicians discuss the risk of ONJ, the signs and symptoms of ONJ, and the risk factors for developing ONJ in patients initiating or receiving treatment with bisphosphonates . The incidence of ONJ may be higher in Asian populations, pointing to a genetic predisposition . Although the most recent guidelines published by the American Association of Oral and Maxillofacial Surgeons suggest to discontinue the oral bisphosphonate for 2 months prior to performing the dental surgery if a patient has been treated for more than 4 years (aiming to restart the bisphosphonate when the bone has healed), there is no evidence to support that this would lower ONJ risk, especially since bisphosphonates stay in bone for years. In fact, the guidelines from the American Dental Association state that the benefit provided by antiresorptive therapy outweighs the low risk of developing ONJ, and that discontinuing bisphosphonate therapy may not lower the risk but may have a negative effect on low-bone-mass treatment outcomes . Patients considering dentoalveolar surgery while taking bisphosphonates should be advised of the risks and alternatives .
74.10.2
Atypical femur fractures
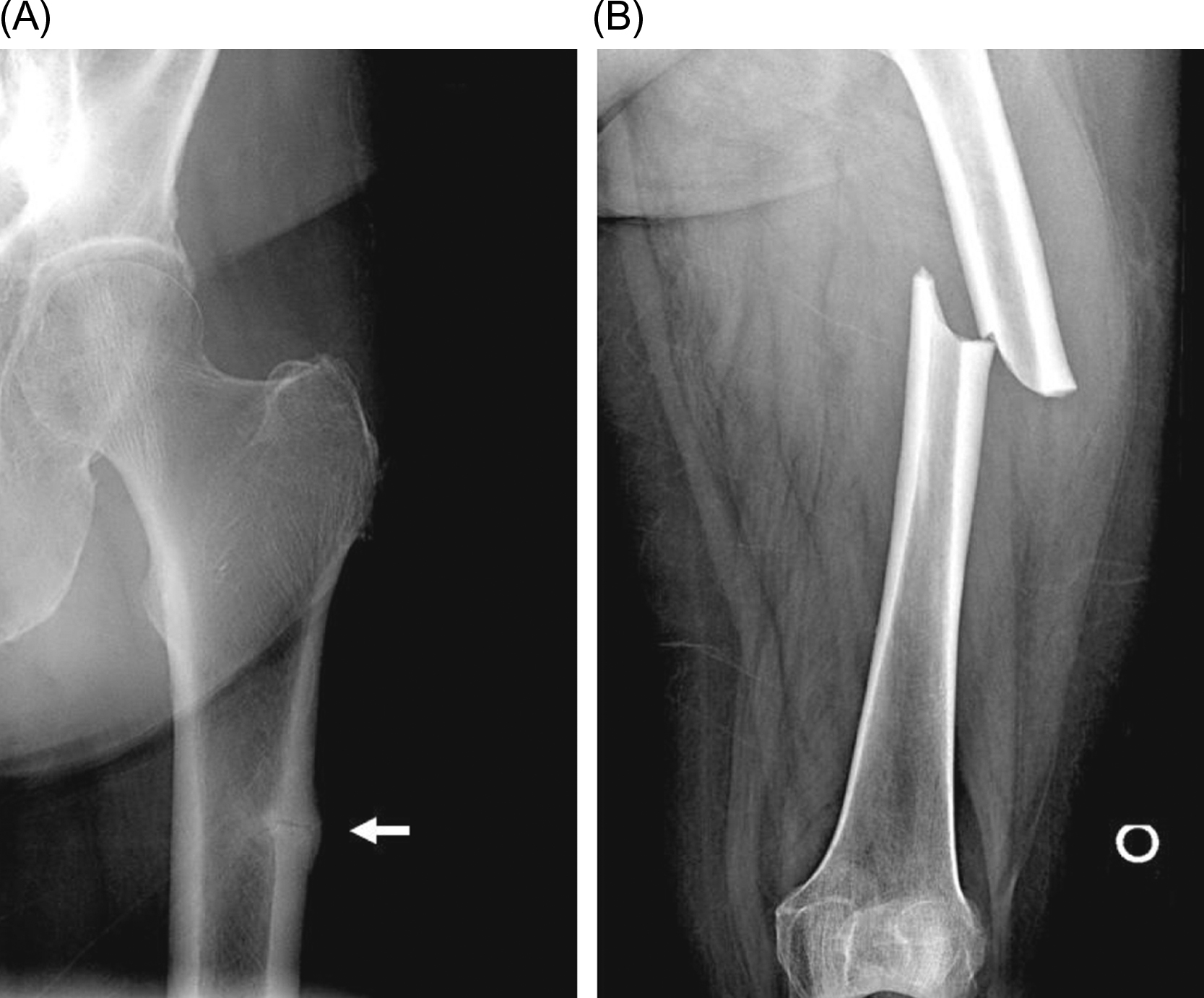
Atypical femur fractures (see also Chapter 81 : Lessons from bone histomorphometry on the mechanisms of action of osteoporosis drugs, Shane, Khosla, and Burr) are unusual subtrochanteric fractures that are associated with minimal or no trauma. These present with prodromal pain in the region of the fracture and have characteristic radiographic findings, including cortical hypertrophy, a transverse fracture pattern, and medial cortical spiking ( Fig. 74.5 ).