Fig. 7.1
This lateral radiograph of the right femur shows lateral, mid-diaphyseal cortical thickening
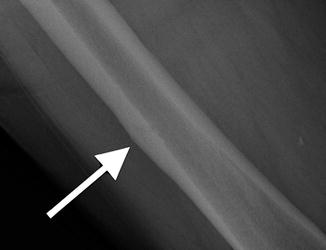
Fig. 7.2
Frontal radiograph better demonstrates some intracortical lucency, possibly an early fracture line
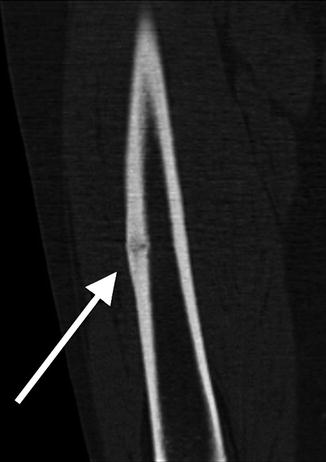
Fig. 7.3
Coronal CT reformation shows lateral cortical thickening with a linear lucency developing. The medial cortex is uninvolved
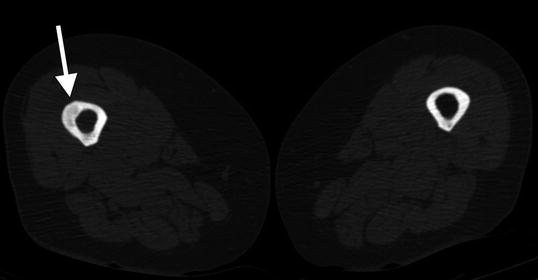
Fig. 7.4
Transverse CT shows thickening and lucency of the right femoral lateral cortex
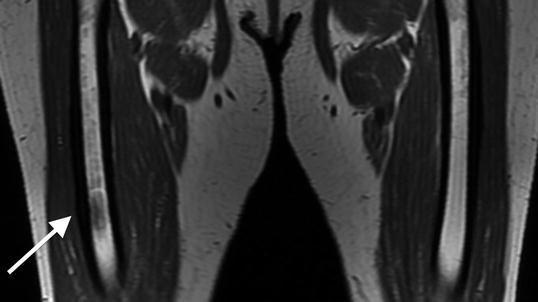
Fig. 7.5
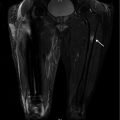
Coronal T1-weighted 1.5 T MRI shows lateral cortical thickening of the right femur with a confluent, decreased marrow signal. The left femur is unremarkable
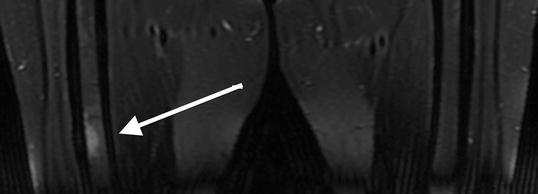
Fig. 7.6
Coronal T2-weighted , fat-saturated 1.5 T MRI shows increased signal intensity of the bone marrow at the non-displaced atypical femoral fracture site
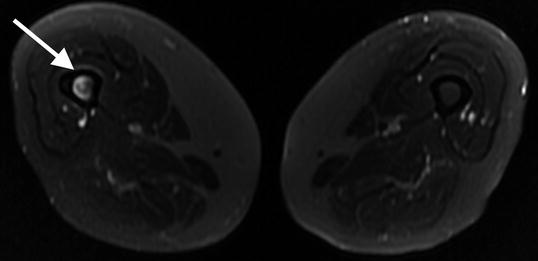
Fig. 7.7
Transverse T2 1.5 T MRI with fat saturation shows increased bone marrow signal

Fig. 7.8
Flow (early) phase of a bone scan . No abnormality is identified
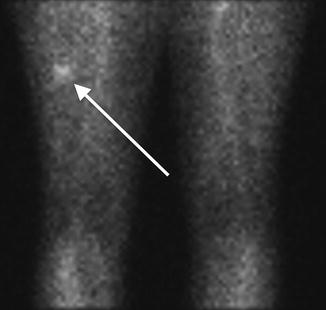
Fig. 7.9
Blood pool phase on bone scan . Increased radiotracer uptake is noted within the right mid-diaphysis
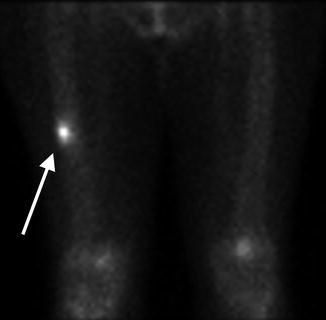
Fig. 7.10
Delayed phase on bone scan shows significantly increased radiotracer uptake. This appearance can be seen in conditions of increased bone turnover, such as osteoblastic metastases and stress fractures, and in this case of non-displaced atypical femoral fracture (AFF)
Conventional Radiography
Atypical femur fractures are defined by their radiographic appearance. Currently, they are defined as transverse fractures arising from the lateral cortex of the femur, inferior to the lesser trochanter. Seventy-nine percent of all atypical fractures occur within 5 cm of the lesser trochanter and, by definition, lie above the supracondylar flare [26]. Early on, there will be thickening (“beaking”) of the medial or lateral aspect of the lateral cortex. With progression, a fracture line will develop and extend medially. There have been several examples of multiple foci developing simultaneously; however, these fractures are rarely comminuted [27–29].
The angle made by the fracture and lateral fe moral cortex is important in elucidating the etiology. Atypical femur fractures comprise only about 1/3 of all subtrochanteric fractures, and, in cases where the fracture angle is shallow, it can be difficult to differentiate bisphosphonate-related fractures from other diagnoses [16]. A fracture angle of 90° ± 15 with associated periosteal beaking has a 90 % specificity for bisphosphonate use [30]. Fatigue fractures will typically be more oblique, in addition to propagating from the medial to lateral cortex [31, 32]. Additional considerations for differential diagnoses are described in conjunction with the figures found at the end of this chapter. Distinguishing bisphosphonate-related injury from classic fractures may become increasingly important in determining prognosis and guiding specific therapy.
Progression to the middle and late stages of the fracture are marked by involvement of the medial cortex and complete displacement of the distal femoral fragment. There is invariably varus angulation, which can help distinguish AFFs from other fracture etiologies.
X-ray evaluation of patients with h ip, thigh, or groin pain on long-term bisphosphonates should include a frontal view of the pelvis and two views of the full length of each femur.
MRI
The MRI characteristics of an atypical femoral fracture are much like any other stress fracture, save for the lateral to medial transverse pattern, as described above. The earliest findings include high-signal periosteal reaction about the lateral cortex with normal marrow signal [33]. MRI is presumed to be the most sensitive modality prior to fracture [33]. Progression is marked by linear low T1 and high STIR or T2 signal within the marrow as the fracture extends medially. Conversely, non-progression or reversal of marrow signal changes on MRI may be a guide for therapeutic efficacy.
MRI may also be of particular benefit in patients with known atypical fracture as a screening technique for the contralateral leg. Risk of contralateral fracture appears to be between 22 and 64 %, and increasing attention is being devoted to prevention of these fractures [20, 21, 24].
A pelvis MRI without contrast protocolled for musculoskeletal purposes will evaluate both hips, the pubic symphysis and sacroiliac joints, and the musculature and tendons of both hips and thus can provide an explanation for pain other than atypical femur fracture. MRI of the entire length of both femora without contrast is also recommended as the next step, as cortical thickening can be subtle, and having the other side for comparison can improve the diagnostic capability of the scan. Furthermore, the marrow lesions can be far down the shaft of the femur, which would be missed if only the hips were imaged. By scanning both femora, asymptomatic lesions can be picked up on the opposite side. Both femora should be scanned simultaneously, thereby not resulting in any increased scanner time, patient discomfort, or cost. And since MRI does not result in any deleterious effects to human tissue, no collateral damage is incurred by scanning both femora. Contrast-enhanced MRI is not needed, as the fin dings are present on the non-contrast MRI scans.
Nuclear Medicine (DXA and Bone Scan)
Two studies under the domain of nuclear medicine have been found to be helpful: dual-energy X-ray absorptiometry (DXA) and bone scintigraphy (or more commonly referred to as a “bone scan”). DXA describes a technique in which X-rays of two different energy levels are used to acquire images of the lumbar spine and hips. The difference in through-transmission of X-rays through these bones can be used to calculate the density of the bones to a high degree of certainty. This is currently the gold standard in the clinical evaluation of bone density and is commonly performed annually to biennially in patients at risk for the development of osteoporosis. For obvious reasons, a large number of patients receiving bisphosphonates likely undergo regular DXA scans. The findings of Atypical femur fractures on DXA appear much like those on conventional radiography, with the exception being that DXA demonstrates a lower degree of spatial reso lution. It should also be highlighted that conventional DXA studies include only 1–2 cm below the lesser trochanter. Given that AFFs typically occur within 5 cm of the lesser trochanter, conventional DXA may miss a large percentage of developing lesions. Many advocate extending the length of the DXA scan in patients on bisphosphonates.
Bone scintigraphy, on the other hand, uses a radioisotope (most commonly technetium-99m-labeled methylene diphosphonate) that shows active bone turnover when imaged with a gamma camera. The basic principle is that labeled methylene diphosphonate (MDP) will attach to phosphate binding sites and radioactivity will accumulate in areas of high turnover. Devices called gam ma cameras can then detect this radiation. A subset of bone scans, the “triple-phase ” bone scans, are imaged at three times: in the first several moments to show perfusion; several minutes later to show “blood pooling,” i.e., inflammation or autonomic dysfunction; and after several hours, at whi ch point any tracer not bound to the bone should have been cleared by the patient’s kidneys. It is during this third, “delayed,” phase that stress fractures and AFFs are most easil y identified. As the comp ound actively seeks out sites of bone turnover, bone scintigraphy can be highly sensitive for developing stress fractures; however, its specificity is extremely limited by a lack of spatial resolution. Atypical femur fractures appear as increased activity in the subtrochanteric region, again, wit h a predilection for the lat eral c ortex.
Differential Diagnosis
While AFFs demonstrate a specific and recognizable pattern, several other conditions may simulate this appearance. Stress fractures of the femur may occur in a subtrochanteric location; however, they most typically begin along the medial cortex and propagate laterally. Pathologic fractures related to underlying osseous lesions may simulate the cortical “beaking” that classically defines a developing bisphosphonate-related fracture. A sinus tract along a region of chronic osteomyelitis may appear similar to a fracture like, with adjacent osseous irregularity. Figures 7.11, 7.12, 7.13, 7.14, 7.15, 7.16, 7.17, 7.18, and 7.19 demonstrate several such mimics.
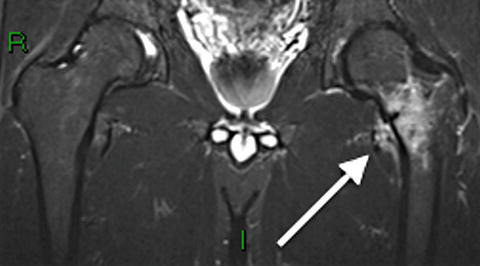
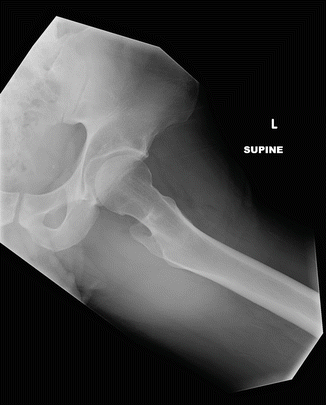
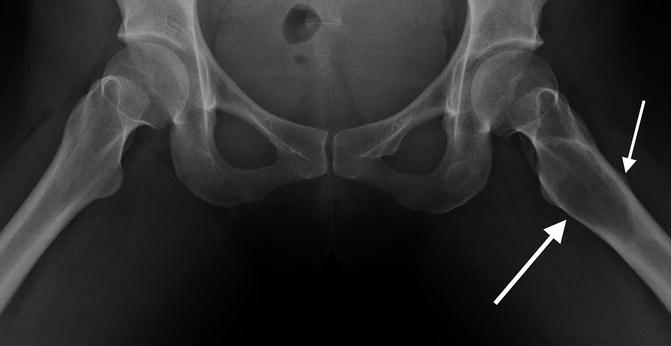
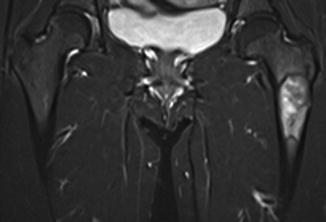
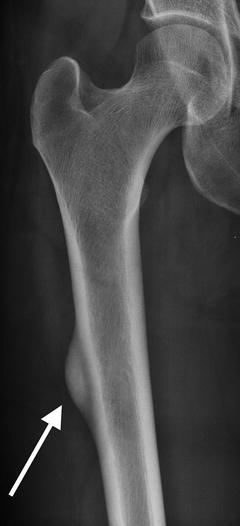
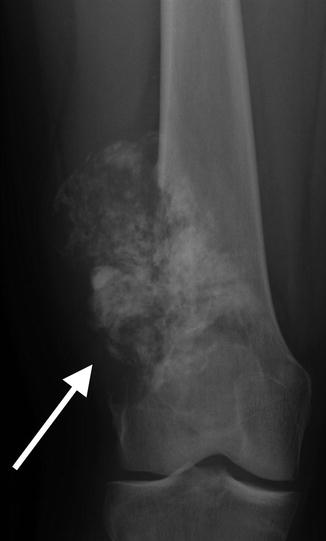
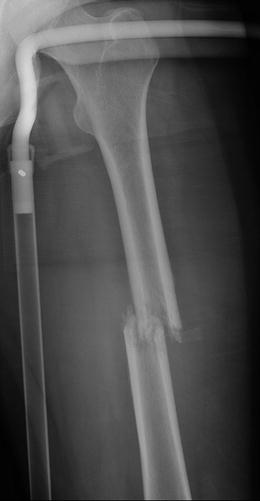
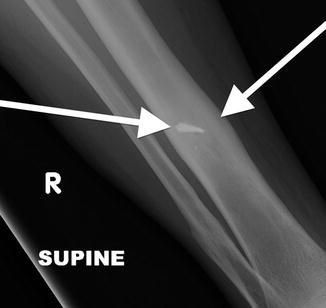
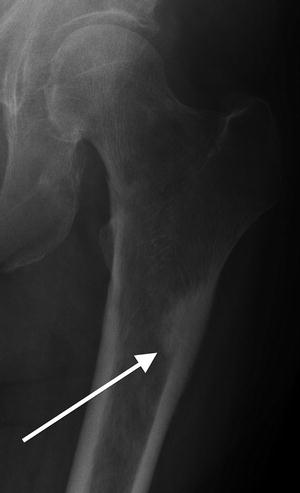

Fig. 7.11
Coronal STIR 1.5 T MRI of a 36-year-old shows a stress fracture of the left femoral neck. A dark fracture line is progressing from medial to lateral and is surrounded by extensive edema signal. Note the predilection for the medial cortex of the femoral neck in contrast to Atypical femur fractures, which begin on the lateral cortex of the subtrochanteric region

Fig. 7.12
Frog-leg lateral radiograph of the same patient in Fig 7.11. Slight medial cortical thickening with sclerotic, non-displaced fracture line oriented perpendicular to the trabecular lines of the femoral neck is characteristic of a stress fracture

Fig. 7.13
AP radiograph of the hips in frog-leg position of a 38-year-old. There is a nearly transverse fracture line progressing from lateral to medial cortex of the left subtrochanteric femur through a lytic lesion in the intertrochanteric and subtrochanteric femur. In this case, the etiology is a pathologic fracture through bone that has been weakened by fibrous dysplasia

Fig. 7.14
Coronal STIR 1.5 T MRI of the same patient as in Fig 7.13 shows a region of increased signal that could mimic marrow edema on first glance. In contrast, this is well-marginated lesion with a narrow zone of transition, with a low signal sclerotic rim, representing fibrous dysplasia. While there is a beaking of the lateral cortex, the cortex is not thickened as one would see in a developing AFF

Fig. 7.15
Apparent lateral cortical thickening in a 41-year-old. This shows an underlying linear lucency and lacks the medial beaking seen in AFFs. This is an osteochondroma, with characteristic continuity of the cortex and medullary space

Fig. 7.16
AP radiograph of the distal femur and knee in a 45-year-old with thigh pain. Large expansile mass with osteoid matrix and cortical bone destruction suggest osteosarcoma, which was proven on biopsy

Fig. 7.17
Radiograph of the femur demonstrates a transverse subtrochanteric fracture in a previously asymptomatic 54-year-old patient. Faintly moth-eaten cortex and intramedullary cavity hints at the patient’s underlying lymphoma which resulted in this pathologic fracture. Note that there is no cortical thickening and the jaggedness of the fracture line

Fig. 7.18
Frontal radiograph of the right tibia demonstrates healed tibia fracture complicated by chronic osteomyelitis. Note the fusiform medial and lateral distal cortical thickening. Can mimic a developing AFF when it occurs in the femur. The presence of a retained drill bit indicates prior surgical intervention (white arrows). Also note a healed distal fibula fracture

Fig. 7.19
Sclerotic non-ossifying fibroma masquerading as lateral cortical thickening. In contrast to the smooth contour of a developing AFF endosteal “beak,” this lesion demonstrates a more acute shoulder at its interface with the cortex as well as an indistinct “brush border”
Screening Considerations
A prodromal pain syndrome only exists in 50–76 % of patients who demonstrate incomplete or developing fractures [24]. Progression from the earliest findings to complete fracture typically takes between 2 and 6 months [34]. Lastly, quantitative risk stratification based on risk factors or by a general tool such as the fracture risk assessment tool (FRAX ) has yet to be fully elaborated. This being said, there is a growing emphasis on finding the best screening test for early fractures. MRI may be an excellent choice for those with the classic prodrome of unrelenting groin pain. For those who are asymptomatic, however, other modalities may prove to be more cost-effective initial screens. One promising suggestion has been to increase the length of femur included in DXA scans, which most bisphosphonate patients undergo routinely [35]. It is unlikely, however, that the routine DXA scan interval is appropriate for the prevention of complete fractures. Nuclear scintigraphy is another possibility with areas of developing fracture appearing similar to a lateral cortical stress fracture. This, however, tends to be positive only in symptomatic patients and may prove to be too insensitive for screening [26]. At our institution, it has become common practice to include the most commonly involved part of the femur in all routine emergency department and outpatient pelvic X-rays as well as all DXA scans . Anecdotally, this has led to a sharp increase in the number of incidentally discovered early AFFs. Regardless of whether initial findings are discovered on conventional radiography or DXA, it is recommended that MRI or CT confirmation should be pursued [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree