BIOSYNTHESIS
IMPORTATION INTO MITOCHONDRIA
The rate-limiting step in steroid biosynthesis is importation of cholesterol from cellular stores to the matrix side of the mitochondria inner membrane where the cholesterol side-chain cleavage system (CYP11A, adrenodoxin, adrenodoxin reductase) is located. This is controlled by the steroidogenic acute regulatory protein (StAR),2,2a the synthesis of which is increased within minutes by trophic stimuli such as adrenocorticotropic hormone (ACTH) or, in the zona glomerulosa, by increased intracellular calcium. StAR is synthesized as a 37-kDa phosphoprotein that contains a mitochondrial importation signal peptide. However, importation into mitochondria is not necessary for StAR to stimulate steroidogenesis; to the contrary, the likelihood
now appears that mitochondrial importation rapidly inactivates StAR.3 The mechanism by which StAR mediates cholesterol transport across the mitochondrial membrane is not yet known.
now appears that mitochondrial importation rapidly inactivates StAR.3 The mechanism by which StAR mediates cholesterol transport across the mitochondrial membrane is not yet known.
StAR clearly is not the only protein that mediates cholesterol transfer across the mitochondrial membrane. Another protein that appears necessary (but not sufficient, at least in the adrenals and gonads) for this process is the peripheral benzodiazepine receptor, an 18-kDa protein in the mitochondrial outer membrane that is complexed with the mitochondrial voltage-dependent anion carrier in contact sites between the outer and inner mitochondrial membranes.4 This protein does not appear to be directly regulated by typical trophic stimuli but is stimulated by endozepines, peptide hormones also called diazepam-binding inhibitors. Endozepines may be regulated by ACTH to some extent, but not with a rapid time course. Thus far, whether or not a direct physical interaction exists between StAR and the peripheral benzodiazepine receptor is not clear.
ENZYMES
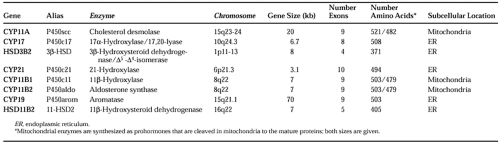
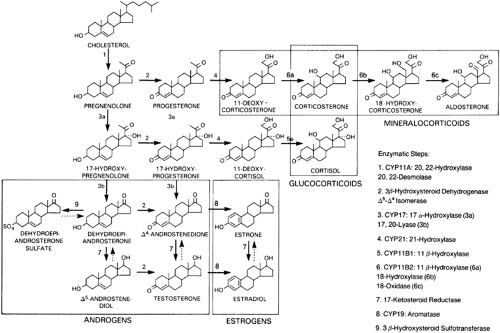
The enzymes required for adrenal steroid biosynthesis comprise two classes: cytochrome P450 enzymes and short chain dehydrogenases (Table 72-2).5 These enzymes are located in either the lipophilic membranes of the smooth endoplasmic reticulum or the mitochondrial inner membrane. As the successive biosynthetic reactions involved in steroidogenesis occur, the adrenal steroids shuttle between the mitochondria and the smooth endoplasmic reticulum (Fig. 72-3).
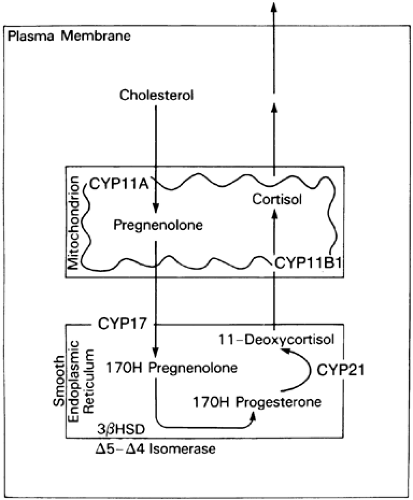 FIGURE 72-3. Biosynthesis of cortisol from cholesterol. The functional names of the steroidogenic enzymes are listed in Table 72-2 and Figure 72-4. |
CYTOCHROME P450 ENZYMES
Cytochrome P450 (CYP) enzymes are responsible for most of the enzymatic conversions from cholesterol to biologically active steroid hormones.5,6 Five P450 enzymes are involved in cortisol and aldosterone synthesis (see Table 72-2). Three—CYP11A (cholesterol desmolase, P450scc; the “scc” stands for “side chain cleavage”), CYP11B1 (11β-hydroxylase, P450c11), and CYP11B2 (aldosterone synthase, P450aldo)—have been localized in mitochondria; two—CYP17 (17α-hydroxylase/17,20-lyase, P450c17) and CYP21 (21-hydroxylase, P450c21)—are located in the endoplasmic reticulum (see Fig. 72-3).
P450 enzymes are membrane-bound hemoproteins with molecular masses of ˜50 kDa. Their name arises from their property of absorbing light at a peak wavelength of 450 nm. These enzymes are located in the membranes of the smooth endoplasmic reticulum and the mitochondrial inner membrane and are encoded by a large superfamily of genes.7 P450s are mixed function oxidases. They use molecular oxygen and reducing equivalents (i.e., electrons) provided by reduced nicotinamide-adenine dinucleotide phosphate (NADPH) to catalyze oxidative conversions of an extremely wide variety of mostly lipophilic substrates.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree