Osteoporosis is characterized by low bone mineral density (BMD) and poor bone structure. Both increase bone fragility and are influenced by bone turnover. Bone turnover comprises formation of new bone matrix by osteoblasts and resorption of old bone by osteoclasts . These opposite activities are coupled in time and space at the level of the bone multicellular unit (BMU). During bone remodeling, bone formation is preceded by bone resorption. During bone resorption, dissolution of bone mineral and catabolism of bone matrix by osteoclasts result in the formation of a resorption cavity and the release of bone matrix components. During bone formation, osteoblasts synthesize bone matrix that fills in the resorption cavity and undergoes mineralization.
Bone mass is determined by the balance between resorption and formation within a BMU and by the number of BMUs. An increased number of BMUs and an imbalance between bone formation and resorption at the BMU result in bone loss and osteoporosis. As the quantity of bone lost at the level of one BMU is small, bone loss is driven by the number of BMUs.
Bone turnover markers (BTMs) are divided into the markers of bone formation and those of bone resorption ( Table 65.1 ). When both processes are coupled and change in parallel, BTMs reflect the overall bone turnover rate. Currently available BTMs do not discriminate between cortical and trabecular bone. An increase in the BTMs is followed by an increase in BMD during growth, but by a decrease in BMD once skeletal maturity is reached.
| Bone formation |
| OC |
| Bone ALP |
| PINP |
| PICP |
| Bone resorption |
| CTX-I |
| NTX-I |
| CTX-MMP, ICTP |
| Helical peptide 620–633 of the α1 chain |
| DPD |
| TRACP5b |
BTMs comprise enzymes reflecting the metabolic activity of bone-forming and bone-resorbing cells (alkaline and acid phosphatases), and bone matrix components released into the circulation during formation or resorption. The International Osteoporosis Foundation and International Federation of Clinical Chemistry defined N-terminal collagen type I extension propeptide (PINP) and serum C-terminal cross-linking telopeptide of type I collagen (CTX-I) as the reference markers of bone formation and resorption, respectively .
65.1
Biochemical markers of bone formation
65.1.1
Serum alkaline phosphatase
Bone alkaline phosphatase (bone ALP) is an ectoenzyme on the outer surface of the cell membrane of osteoblasts. It is partly released into the circulation. Bone ALP and liver ALP constitute about 95% of the total ALP activity in human serum. They are isoforms of the tissue nonspecific ALP encoded on chromosome 1 and differ by posttranslational modifications. Other isoenzymes include intestinal ALP, placental ALP, and germ cell ALP (present in serum of patients with seminoma) .
Bone ALP regulates bone mineralization. It provides inorganic phosphate from pyrophosphate and phosphomonoesters and hydrolyzes pyrophosphate (mineralization inhibitor) for the hydroxyapatite synthesis . In mice, inactivation of the TNSALP gene resulted in reduced longitudinal growth, short growth plates, hypomineralized areas in bones and reduced survival . Inactivating mutations of the TNSALP gene in man cause hypophosphatasia.
Bone ALP can be evaluated by manual or automated immunoassay, enzyme activity after wheat germ precipitation or high-performance liquid chromatography. Immunoassays for bone ALP measurement use monoclonal antibodies that preferentially recognize the bone isoform . However, they exhibit noticeable cross-reactivity with the liver ALP (20%).
65.1.2
Osteocalcin (bone Gla protein)
Osteocalcin (OC) (49 amino acids) is a noncollagenous protein of the bone matrix. It contains three gammacarboxyglutamic residues, products of gammacarboxylation of glutamic residues stimulated by vitamin K . It is synthesized by osteoblasts and odontoblasts and is specific for bone and dentin. Data on the function of OC are discordant. OC knock-out mice had higher cortical thickness and higher bone formation . OC may be a signal from bone matrix–stimulating migration and adhesion of osteoclasts . OC regulates energy metabolism, probably acting via its receptor GPRC6A .
OC knock-out mice had higher glycemia, lower insulin level, impaired glucose tolerance, and insulin resistance . The undercarboxylated fraction (ucOC) is suggested to be the active form of OC. Subjects with lower OC or ucOC levels had higher levels of glucose, glycosylated hemoglobin and insulin, greater insulin resistance, and more severe metabolic syndrome . OC might be also involved in metabolic pathways regulating brain development and function, testosterone secretion, and fertility .
OC is incorporated into the bone matrix, but a fraction of newly synthesized OC is released into the blood where it is measured by immunoassay . As blood OC is rapidly cleared by the kidney , OC and its fragments accumulate in patients with renal failure. Serum OC is a specific marker of bone formation and is correlated with histomorphometric bone formation parameters . Serum OC level is low in conditions characterized by low bone formation and high resorption, for example, in multiple myeloma . Falsely low values may result if hemolysis is present.
The bulk of serum OC is released during bone formation . The contribution of bone resorption to circulating OC is unimportant if renal function is normal . Serum OC level is higher in conditions characterized by high bone turnover, both physiological (puberty) and pathological ones [hyperthyroidism, primary hyperparathyroidism (PHPT), acromegaly, bone metastases] . Serum OC is low in hypothyroidism, hypoparathyroidism, diabetes mellitus, Cushing’s disease, glucocorticoid-treated patients, multiple myeloma, and malignant hypercalcemia.
In adults, intact OC represents one-third of serum OC immunoreactivity . One-third is an N-terminal-mid-molecule fragment and one-third comprises minor fragments. At room temperature a large fraction of intact OC is converted into the N-terminal fragment. These findings explain the wide scatter of individual values obtained by using different antibodies . Thus serum for the intact OC assay should be promptly centrifuged and frozen, whereas the N-mid-fragment remains stable for some period of time at room temperature.
65.1.3
Procollagen type I propeptides
C-terminal collagen type I extension propeptide (PICP) and PINP are cleaved during the posttranslational extracellular processing of type I collagen molecules before assembly into fibrils. Both circulate in blood. Type I collagen is the most abundant protein in bone (90% of bone matrix). Type I collagen is not specific for bone; it is also synthesized by other connective tissues, particularly skin. As their metabolism is slower than that of bone, serum PICP and PINP originate mainly from bone. However, active fibrosis of lungs, liver, or heart may contribute significantly to the serum levels of PICP and PINP . The quantities of PINP and PICP are equimolar to that of collagen deposited in bone matrix. PICP is a globular molecule stabilized by the disulfide bonds between the chains . Unstable trimeric PINP is promptly transformed into stable monomers. There are two circulating forms of PINP: in vivo cleaved trimeric peptide and low-molecular-weight peptides of α1 and α2 chains .
After natural and surgical menopause, PINP increases similarly to bone ALP and OC . Serum PINP has a small circadian variability and does not necessitate blood collection in the fasting status in the morning . Therefore PINP seems to be useful for monitoring antiosteoporotic therapy . In particular, serum PINP increases promptly after the initiation of bone formation–stimulating therapy and its early increase is significantly correlated with the long-term increase in BMD .
After menopause, PICP increases less than other BTMs . In osteoporotic patients, PICP correlated weakly with histomorphometric bone formation parameters . PICP decreased during antiresorptive therapy and increased during treatment with parathyroid hormone (PTH) . However, its utility for clinical management of postmenopausal osteoporosis is limited. In contrast, its level is significantly correlated with the rate of longitudinal growth in children . It also is useful for monitoring treatment with growth hormone (GH) in children with GH deficiency and in children with osteogenesis imperfecta .
The different sensitivities of both propeptides may be due to the different sites and regulation of their catabolism. Both are degraded by the endothelial cells of the liver. However, PICP is taken up by the mannose receptor (induced by IGF-I and thyroid hormones) and PINP by the scavenger receptor (not under endocrine control) .
65.2
Biochemical markers of bone resorption
65.2.1
Fasting urinary calcium, hydroxyproline, and hydroxylysine glycosides
Calcium released during bone resorption contributes to urinary calcium excretion. Urinary creatinine-corrected calcium is inexpensive and detects markedly increased bone resorption. However, as it depends on calcium intake and renal calcium handling, it lacks sensitivity.
Hydroxyproline (Hyp) is produced by posttranslational hydroxylation of proline . Hyp is released mainly from bone collagen, but the C1q fraction of complement also contains a high amount of Hyp. Hyp released during breakdown of proteins is not reutilized. Nearly 90% of Hyp filtered in the kidney is reabsorbed by tubular cells, then, oxidized and degraded . Only 10% of Hyp is excreted in urine and only a part of this is of bone origin. Urinary Hyp is strongly influenced by collagen in the diet. Urinary Hyp is poorly correlated with bone resorption assessed by other methods and its use has been abandoned.
Hydroxylysine (Hyl) is unique to collagen and similar proteins. Posttranslational lysyl hydroxylation is followed by glycosylation. Galactosyl-Hyl (Gal-Hyl) is present mainly in bone, whereas glucosyl-Gal-Hyl is found mainly in skin and C1q. They are not metabolized, not reutilized in protein synthesis and not influenced by nutrition. Urinary Gal-Hyl excretion increases with age and after menopause . In patients with Paget’s disease, bisphosphonates decreased urinary Gal-Hyl excretion . In postmenopausal women with prior fractures, urinary Gal-Hyl excretion was 30% higher versus women without fracture . The disadvantage of Hyl and Gal-Hyl is the absence of a rapid and convenient assay.
65.2.2
Plasma tartrate–resistant acid phosphatase
Tartrate–resistant acid phosphatase comprises enzymes that are synthesized in bone, spleen, and lungs . Its serum level is higher than in plasma, because it is released during the clotting process. Levels are high in the presence of hemolysis. Other acid phosphatases are present in other tissues (e.g., prostate). The subform b of the isoenzyme 5 (TRACP5b) is most specific to osteoclasts . At room temperature, it loses >20% of its activity per hour. It is unstable in frozen samples. In blood, it may be bound to α2-macroglobulin that influences its enzymatic activity and deteriorates its binding with assay antibodies .
TRACP5b is the only available marker reflecting the number of osteoclasts. Its level is high in diseases with high bone resorption (hyperparathyroidism, osteoporosis, multiple myeloma, Paget’s disease, bone metastases) and in type II osteopetrosis characterized by a high number of osteoclasts unable to resorb bone . Its plasma activity is not influenced by the function of the liver or kidneys . After the menopause, TRACP5b increases less than other bone resorption markers. Data on fracture prediction by TRACP5b are limited . TRACP5b activity decreases during antiresorptive therapy, but less so than other BTMs . Serum TRACP5b was stable or increased in patients treated with a cathepsin K (catK) inhibitor, odanacatib, which does not decrease the number of osteoclasts .
65.2.3
Collagen pyridinium crosslinks and associated type I collagen peptides
Pyridinoline (PYD) and deoxypyridinoline (DPD) are nonreducible pyridinium crosslinks in the mature collagen. PYD is found mainly in articular cartilage, while DPD is present in minute amounts in this tissue . Both are present in tendon, dentin, and aorta but absent from the skin which is rich in type I collagen. They are generated from hydroxylysyl and lysyl residues . The first step is regulated by lysyl oxidase; the further steps are spontaneous and time-dependent. PYD and DPD are formed during the extracellular maturation of fibrillar collagen and create interchain bonds stabilizing the molecule in the matrix. Both are released during the breakdown of mature cross-linked collagen. Their levels in bone, blood, or urine are not influenced by dietary sources nor degradation of newly synthesized collagen. They are released into the blood after bone resorption and not reused during collagen synthesis.
As formation of PYD and DPD is time-dependent, their content increases with age of bone. It is higher in old interstitial bone than in younger osteonal bone . It is lower in old individuals, who have higher activation frequency, than in middle-aged ones. Therefore DPD excretion does not measure the quantity of resorbed bone.
DPD and PYD are excreted in urine as free and peptide-bound forms. The proportion of free forms is lower in serum than urine (16%–20% vs 40%), and renal clearance is fourfold higher for free than for peptide-bound crosslinks . Crosslink-containing peptides may be taken up by tubular cells where they are cleaved to free forms, then reexcreted into the tubular lumen and excreted in urine. The free crosslink fraction in urine decreases with the acceleration of bone turnover which suggests that the conversion of peptide-bound to free crosslinks is saturable .
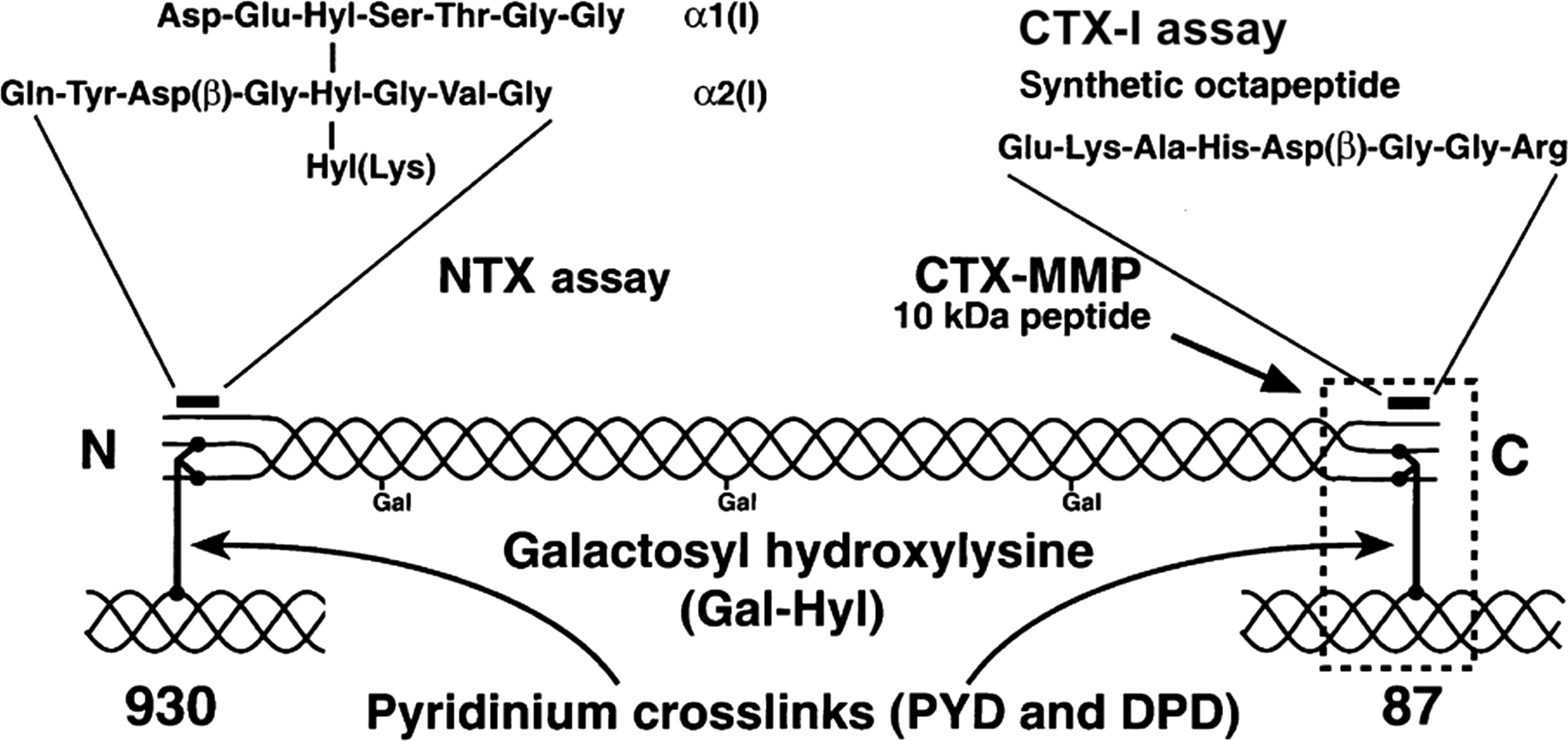
DPD is a valid bone resorption marker. Urinary DPD level is correlated with bone turnover which is measured by calcium kinetics and osteoclast surface (histomorphometric parameter of active bone resorption) . Bisphosphonates rapidly decrease urinary DPD excretion . Immunoassays measure free DPD (fDPD) in urine . Immunoassays are available for a number of the peptide products of degradation of type I collagen. The telopeptides from the C- (CTX-I) and N-terminal (NTX-I) regions are released; the CTX-I is first formed in the alpha configuration and then isomerizes to the beta configuration (α and β-CTX-I). Other fragments are released during bone resorption including an α1 helicoidal peptide 620–633 and a large C-terminal telopeptide fragment generated by matrix metalloproteinases (CTX-MMP, older name: cross-linked carboxyterminal telopeptide of type I collagen, ICTP) ( Fig. 65.1 ). They can be measured in urine (CTX-I, NTX-I), plasma (CTX-I, ICTP), and serum (all four).
Bone resorption markers show a circadian rhythm . Urinary excretion of PYD, DPD, and that of cross-linked telopeptides have similar diurnal variability. They peak in the second half of the night and reach a nadir in the afternoon. The time of the peak is similar for different bone resorption markers, whereas the amplitude is higher for CTX-I (40%–60% of the mean) than for other BTMs (10%–35%). Thus the different markers differ in bone specificity or metabolic rate. Fasting lowers, whereas feeding increases, the amplitude of circadian variation of serum CTX-I . Thus circadian variation has a substantial impact over the BTM levels, which indicates the importance of standardizing the time of sampling.
Serum and urine NTX-I is measured by an immunoassay using an antibody against an epitope on the α2 chain of type I collagen . Its amino acid sequence contains lysine involved in a trivalent cross-linking site. Pyridinium crosslink is not necessary for the reactivity. Digests of skin collagen also react with NTX-I . NTX-I is stable at room temperature and during up to 3 thaw–freeze cycles . NTX-I was studied for the prediction of bone metastases in patients with neoplasms and for monitoring the efficacy of antiresorptive treatment .
Type I collagen α1 helicoidal peptide 620–633 originates from the helical part of collagen . Its urinary excretion and that of CTX-I are correlated. Both markers increase similarly after the menopause and decrease similarly during antiresorptive treatment .
The ICTP and CTX-I levels reflect activities of enzymes involved in the degradation of collagen. ICTP level reflects the activity of MMPs: collagenases (MMP-1,-2,-13,-14) and gelatinases (MMP-2,-9), while CTX-I level reflects the activity of catK which is an osteoclast-specific cysteine protease .
MMPs produce ICTP which is degraded by catK to CTX-I . The ICTP epitope requires a trivalent crosslink, including two phenylalanine-rich domains of the α1 chain of type I collagen . The ICTP level increases modestly after the menopause. It decreases during hormone replacement therapy (HRT), but less so than other bone resorption markers . During treatment with a catK inhibitor, ICTP increases because it cannot be degraded . Then, after treatment cessation, ICTP levels decrease. In patients with pycnodosystosis (mutation in the catK gene) serum ICTP is high, whereas CTX-I level is low . This supports the suggestion that, in healthy persons, a large part of ICTP is degraded by catK, whereas this mechanism is absent in pycnodysostosis.
MMPs induce cytokines that degrade the basement membranes of blood vessels and facilitate spread of metastases . Serum ICTP is higher in patients with bone metastases from lung or prostate cancer and in those with multiple myeloma with osteolytic lesions . ICTP levels decrease in patients with bone metastases receiving bisphosphonates .
65.2.4
C-terminal cross-linking telopeptide isomerization
CTX-I is measured by an immunoassay with an antibody raised to an epitope on the α1 chain of type I collagen. Its amino acid sequence ( 1207 G K AH DG GR 1214 ) contains lysine (K), part of a trivalent crosslink and a sequence aspartate-glycine (DG). β-Isomerization and racemization of the native βL sequence generate three age-related isomers: isomerized (βL), racemized (αD), and isomerized/racemized (βD) form ( Fig. 65.2 ). They are generated in bone matrix in the sequence αL→βL→βD→αD during the aging and maturation of collagen . CTX-I is bound to three α chains: two nonhelical C-terminal telopeptides on the α1 containing the GKAHDGGR sequence and one helical part of collagen closer to the N-terminus. The β-isomerization and racemization occur on the DG sequences on the C-terminal telopeptides. The αL-CTX-I reflects the resorption of newly formed bone. αL-, βD-, and αD-CTX reflect the degradation of aged bone, old bone, and very old bone, respectively .
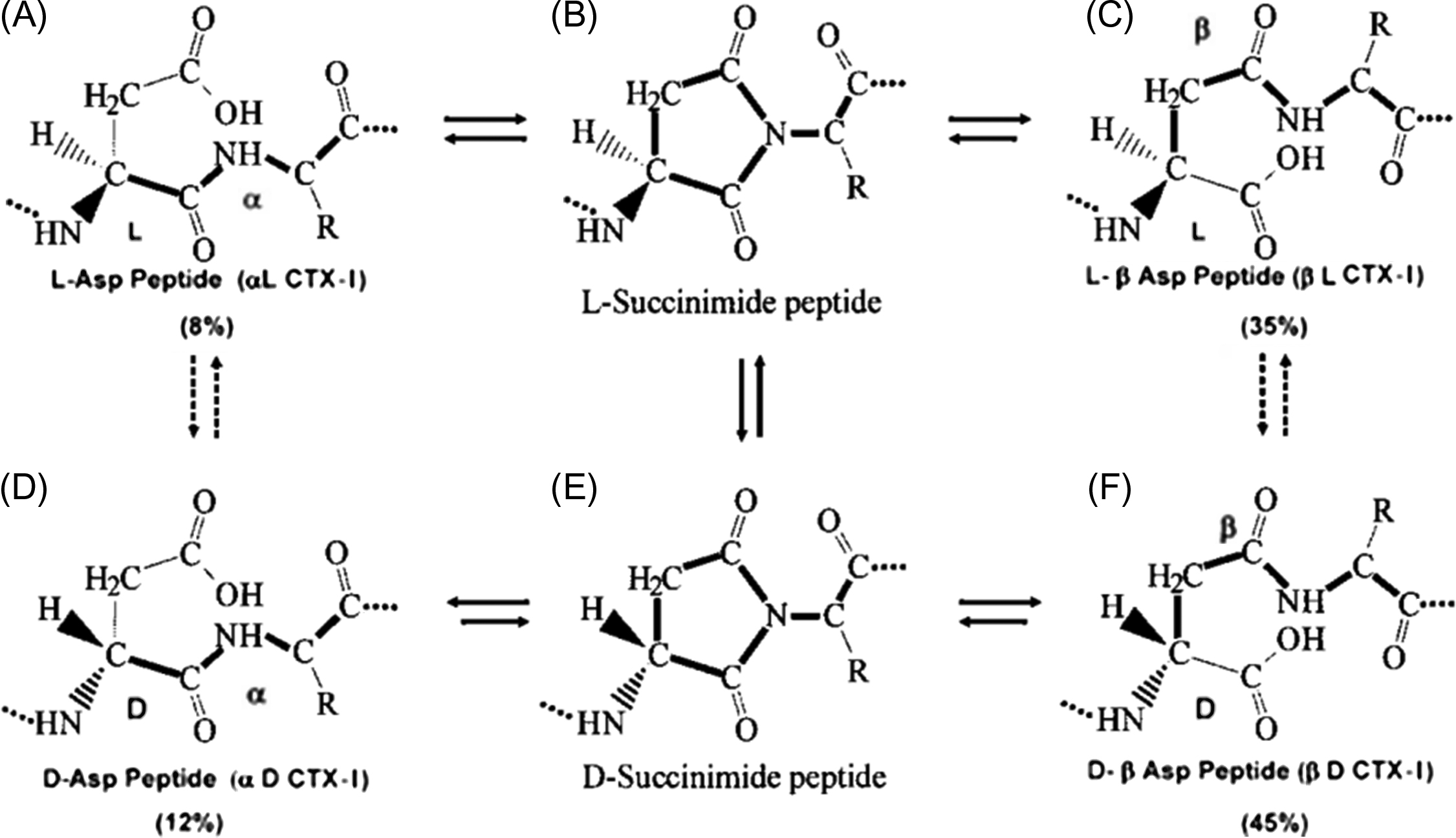
Lower isomerization/racemization of the DG sequence confirmed by high urinary αL/βL and αL/αD ratios is found in woven pagetic bone . This low β-isomerization may reflect a defect of the collagen fibers. After bisphosphonate treatment the α/β-CTX-I ratio is normalized in line with the progressive replacement of woven bone by lamellar bone with normal β isomerization of collagen . In other diseases characterized by high bone turnover the α/β-CTX-I ratio is in the normal range .
Bone metastases are characterized by rapid bone turnover in localized areas of bone and high levels of bone resorption markers. NTX-I reflects mainly the mass of resorbed bone. CTX-I isoforms may reflect bone turnover rate and a defect of β-isomerization. In men with prostate cancer and bone metastases the αL/αD ratio between the urinary degradation products of the recent and the oldest forms of collagen was increased .
The immunoassays use antibodies against two α-CTX-I chains typical for native collagen or two β-CTX-I chains typical for aged collagen. In women with bone metastases due to breast cancer, α-α-CTX-I level was high versus other CTX-I forms and decreased rapidly during antiresorptive treatment . β-CTX-I is not useful in the setting of malignancy.
65.3
Analytical and preanalytical variability
The analytical variability, assessed by the intra- and interassay coefficients of variation (CV), depends on the BTM, the assay and the technician’s expertise . BTMs are measured mainly by radioimmunoassay, immunoradiometric assay, enzymatic immunoassay, or chemiluminescence. The use of monoclonal antibodies permits their specific measurements. Automated analyzers permit a rapid, convenient, fully automated and precise measurement of BTMs in research projects and clinical practice .
The preanalytical variability comprises two groups of factors: controllable factors and factors that are not easily modified ( Table 65.2 ).
| Source of variability | Comment |
|---|---|
| Determinants which can be controlled | |
| Major impact | |
| Circadian variation | Important for bone resorption, especially for CTX-I. Blood should be collected after overnight fast before 10.00 a.m. |
| Feeding status | |
| Moderate or minor impact | |
| Menstrual cycle | Minor fluctuations. More data required |
| Season | May be important in elderly home-bound or institutionalized individuals, minor impact in community-dwelling normally active population |
| Lifestyle (smoking, drinking, dietary habits) | More data required. Main findings: higher bone resorption in smokers, lower bone formation in heavy drinkers. Avoid binge drinking on the day prior to blood collection |
| Exercise |
|
| Determinants which cannot be easily controlled | |
| Major impact | |
| Age | Most important factors : BTM levels are higher in young men versus premenopausal women and lower in older men versus postmenopausal women |
| Gender | |
| Menopausal status | |
| Day-to-day variation | Strong and variable impact, intraindividual coefficient of variation varied from 4% to 20% between the subjects. Data obtained mainly in the short-term studies, more studies on the long-term variation required |
|
|
| Lactation | All BTMs elevated |
| Renal failure |
|
| Conditions characterized by accelerated bone turnover—increased levels of bone resorption and bone formation markers | |
| Increased levels of the markers of bone resorption and bone formation proportionally to the severity of the disease |
| Paget’s disease | Substantial increase in all BTMs, especially total and bone ALP. BTM levels higher in polyostotic disease |
| Metastatic bone disease | Increased NTX-I, CTX-MMP and native, nonisomerized α-α-CTX-I. Higher BTM levels associated with high risk of skeletal complications, disease progression, resistance to treatment and death |
| Vitamin D deficiency and secondary hyperparathyroidism | Increased levels of the markers of bone resorption and bone formation mainly in the home-bound and institutionalized seniors |
| Fracture | Increased BTM levels during the first months. The degree of increase dependent on the surface of the fracture. After a major fracture (e.g., hip), BTM may be elevated for 1 year |
| Bariatric surgery | Increase in bone resorption and formation. The extent of the increase varies according to the method of surgery |
| Long-term immobilization | Higher rise in bone resorption than in bone formation |
| Critically ill patients | Substantial increase in bone resorption and bone formation |
| Conditions characterized by the dissociation of bone turnover—bone resorption increased to various extent, bone formation normal or decreased | |
| Multiple myeloma |
|
| Spinal cord injury | Increased bone resorption, mainly during the first year after the injury. Inconsistent data on bone formation |
| Anorexia nervosa | Increased bone resorption and low bone formation in untreated patients, progressive, but partial, improvement during the treatment |
| Humoral hypercalcemia of malignancy | Resorption—substantial increase of all markers. Formation—normal, slight decrease or mild increase |
| Rheumatoid arthritis | Acute phase: bone resorption—increased, bone formation—normal. BTMs vary according to the phase and therapy |
| Incidentaloma | Decreased OC concentration, other BTMs—normal |
| Cushing’s disease | Bone resorption—slightly elevated. OC—decreased. Other bone formation markers—normal |
| Crohn’s disease | Inconsistent data depending on the phase of the disease and the treatment |
| Malabsorption syndrome | Data vary according to the severity of underlying disease |
| Liver disease | Acute phase—bone resorption increased. Late (fibrotic) phase—PINP and PICP increased due to the increased synthesis and impaired degradation of type I collagen |
| HIV infection | Strongly dependent of the phase of the disease, treatment, comorbidities and lifestyle |
| Conditions characterized by low bone turnover—decreased levels of the markers of bone formation and of bone resorption | |
| Hypothyroidism | |
| Hypoparathyroidism | |
| Growth hormone deficiency | |
| Diabetes mellitus | |
| Medications | |
| Vitamin D and calcium | Decrease in bone formation and in bone resorption |
| Corticosteroids (oral and parenteral) | Formation—rapid decrease in OC, followed by that in PINP; inconsistent data on Bone ALP. Resorption—short-lasting increase at the beginning. Data on BTM vary according to the dose, duration of treatment, and underlying disease |
| Inhaled corticosteroids | OC—decreased. No effect on other BTMs |
| Hormonal contraceptives | Estrogens—lower levels of all BTMs. Progesterone derivatives—slight increase in bone formation markers |
| Aromatase inhibitors | Moderate increase in all BTMs |
| Gonadoliberin antagonists | Increase in bone resorption and formation markers. Used in the treatment of endometriosis in premenopausal women and in the treatment of prostate cancer in older men. |
| Antiepileptic drugs | Inconsistent data obtained in small groups |
| Thiazide diuretics | Mild decrease in BTM levels |
| TZD | Decrease in PINP in some studies. Inconsistent data for other BTMs, partly related to the duration of the treatment |
| Vitamin K antagonists | Decrease in total OC level associated with an increase in the undercarboxylated fraction of OC. No effect on other BTMs |
65.3.1
Controllable factors
Controllable factors having a strong impact on the BTM levels are circadian variability and feeding status. Bone resorption has its peak in the second half of the night and its nadir in the late afternoon . Food intake strongly decreases bone resorption, as reflected by serum CTX-I . This postprandial decrease in CTX-I is mediated by glucagon-like peptide 2, the synthesis of which is induced by food, mainly glucose . Fasting reduced the morning drop in CTX-I . The postprandial decline in CTX-I is smaller after jejunostomy, which may contribute to low BMD in patients with short bowel syndrome . The variability of bone formation markers is low. The amplitude is 5%–15% of the mean . Some factors modify the amplitude of BTMs. After menopause the amplitude was higher in osteoporotic women (vs those with normal BMD) but lower in bisphosphonate-treated women . Other factors (endogenous cortisol, day–night cycle, bed rest, season, calcium intake) have a weak effect on the circadian bone turnover variation.
Menstrual variability has a minor impact on BTMs . The impact of season is mild and rarely significant in healthy adults . High BTM levels are found in winter in home-bound or institutionalized elderly with poor mobility, low sunlight exposure, and vitamin D deficiency .
Lifestyle has a limited impact on BTM levels. Smokers have high bone turnover (mainly bone resorption) . Alcohol ingestion (both after a single drink and with regular consumption) lowers bone turnover, especially bone formation, leading to a dissociation of bone turnover . BTM levels may normalize during abstinence. In postmenopausal women, leisure physical exercise has no significant effect on BTM levels .
65.3.2
Factors that are not easily modified—factors having major impact
Among the factors that are not controllable, age, sex, menopausal status, and day-to-day variation have the strongest impact on the BTM levels.
During the first two trimesters of pregnancy, bone resorption increases, whereas bone formation remains low . In the third trimester, bone resorption and bone formation markers are high. During the lactation period, all BTMs (resorption, formation) are increased . This high bone turnover results in bone loss and, rarely, osteoporosis .
Most BTMs (OC, PINP monomer, CTX-I) are cleared by the kidney. Their blood levels increase proportionally to the loss of renal function and do not reflect the actual bone turnover rate. By contrast, intact trimeric PINP, bone ALP, and TRACP5b are cleared by the liver. Their blood levels are not influenced by renal function and reflect the bone turnover rate in patients with renal failure. Various forms of renal osteodystrophy (osteomalacia, secondary hyperparathyroidism, adynamic bone disease) are characterized by abnormal bone turnover. Given the differences in the therapeutic approach, it is important to assess the bone turnover rate when a bone biopsy is not feasible . High serum levels of bone ALP, intact PINP, and TRACP5b show a satisfactory ability to identify patients with renal failure and high bone turnover (secondary hyperparathyroidism) ; low levels are associated with low bone turnover, typical of adynamic bone disease. The interpretation of the BTM levels in patients with renal failure should also account for other factors influencing BTM levels (vitamin D status, coexisting diseases, treatment).
65.3.3
Factors that are not easily modified—conditions characterized by high bone turnover
BTMs are increased in diseases characterized by higher bone turnover such as thyrotoxicosis, PHPT, acromegaly . The more severe the disease, the higher are the BTM levels. In severe PHPT, BTM levels are markedly elevated, whereas in asymptomatic PHPT, BTM levels are at the upper limit of the normal range . In Paget’s disease, BTMs are higher, mainly total and bone ALP, PINP, and bone resorption markers . BTM levels are higher in polyostotic versus monostotic forms. During treatment, a decrease in the BTM levels is followed by the clinical improvement .
BTM levels are markedly higher in patients with bone metastases, particularly in postmenopausal women with breast cancer . ICTP and α-α-CTX-I seem to be strongly influenced by the bone involvement . Conversely, the BTM levels can be close to the normal range in patients receiving an efficacious anticancer treatment .
Vitamin D status depends on the season, aging of the skin, nutrition, and renal function . Therefore bone metabolism and BTMs depend on the vitamin D and calcium status mainly in the elderly. During winter the concentration of 25-hydroxycholecalciferol (25OHD) is lower and levels of PTH and BTMs are higher, especially in older people . They are particularly high in the housebound and institutionalized elderly who have very low 25OHD levels.
BTM levels are influenced by recent fracture, including vertebral fracture ( Fig. 65.3 ). During the first hours after fracture the OC level decreases due to stress-induced cortisol secretion . Then, bone formation and resorption markers increase promptly and significantly (30%–100%) reflecting the fracture healing . A recent clinically silent vertebral fracture can also increase BTM levels. The larger the cross-sectional surface area of the broken bones, the greater and more long-lasting is the increase in BTM concentrations. Surgical fixation of the fracture also increases the metabolically active surface. BTM levels are increased for at least 4 months after fracture then decrease but may remain elevated for even 1 year .
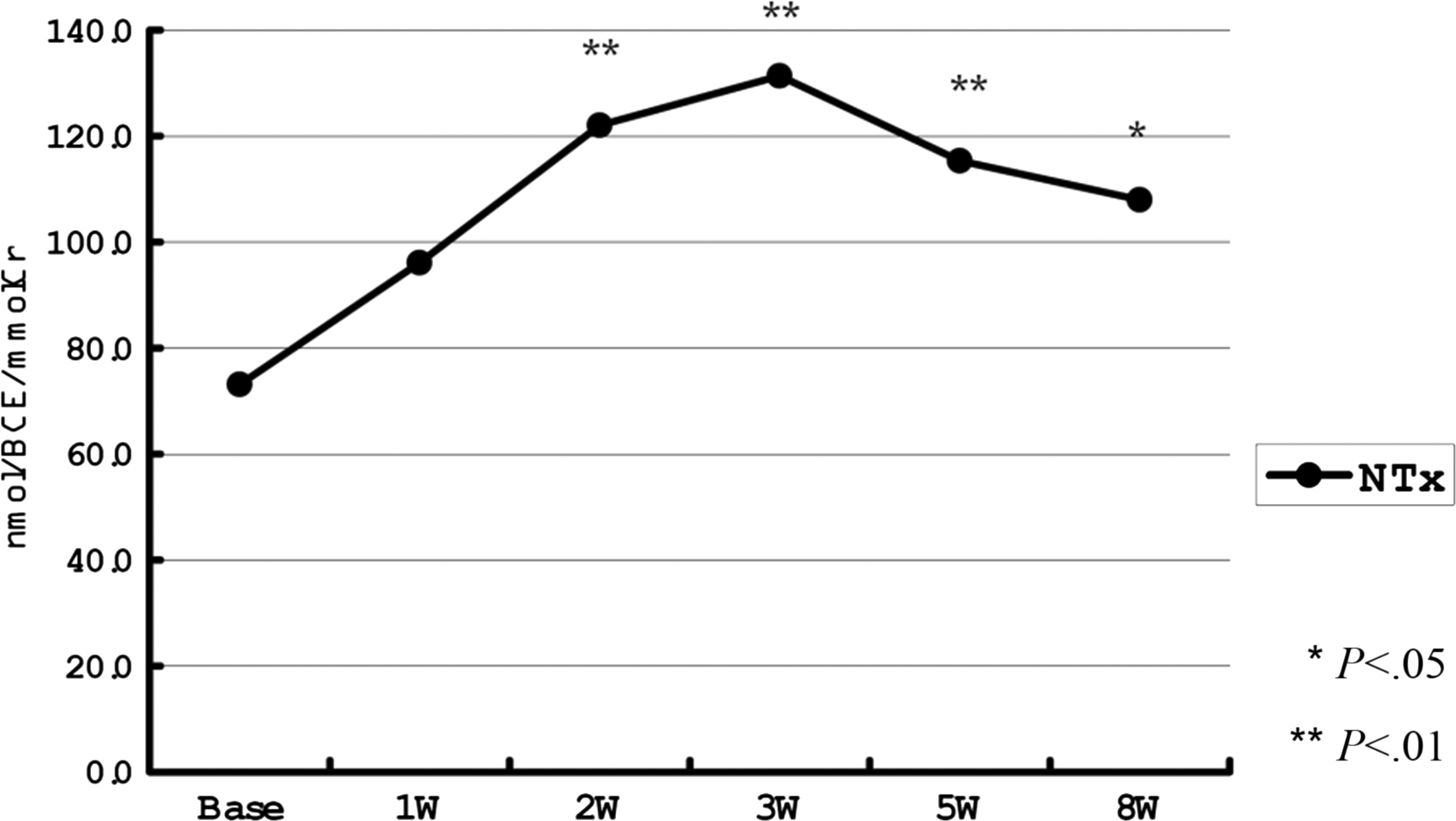
Bariatric surgery used in the treatment of morbid obesity is followed by a rapid increase in the bone turnover rate (both formation and resorption) leading to rapid bone loss . Roux-en-Y gastric bypass seems to result in a greater increase in BTM levels and greater bone loss compared to adjustable gastric banding or sleeve gastrectomy .
Increased BTM levels, mainly those of bone resorption, are found in the institutionalized elderly and patients with chronic diseases associated with limited mobility (dementia, stroke, hemiplegia, Parkinson’s disease) .
Critical illness requiring intensive care admission is associated with higher BTM levels (both formation and resorption) reflecting accelerated bone turnover .
Bone resorption is increased and bone formation is lower in diseases characterized by the dissociation of these processes (multiple myeloma, spinal cord injury, anorexia nervosa, humoral hypercalcemia of malignancy, rheumatoid arthritis, incidentaloma, Crohn’s disease, Cushing’s disease, malabsorption syndrome, liver disease) . The extent of the change in the BTM levels depends on the severity of the disease, for example, it is more evident in polyostotic multiple myeloma . BTM levels depend on the phase of the disease (e.g., rheumatoid arthritis, liver diseases, spinal cord injury) or on the underlying disease (e.g., malabsorption syndrome) . They also depend on the treatment and, usually, partly normalize during clinical improvement .
In HIV infection, low bone formation may be due to a direct effect of the virus on osteoblasts, whereas inflammatory cytokines stimulate bone resorption . Smoking, alcohol abuse, sedentary lifestyle, and comorbidities (hepatitis C) are more frequent in these patients and may influence BTM levels. Bone resorption can be stimulated by drugs used in the treatment of the HIV infection, mainly nucleoside reverse transcriptase inhibitors (e.g., tenofovir) and protease inhibitors . Tenofovir may also have a direct toxic effect on osteoblasts . In addition, some HIV-infected patients receive glucocorticoids.
In diseases characterized by low bone turnover (hypoparathyroidism, hypopituitarism, GH deficiency), bone formation and resorption may be mildly decreased . The more severe the disease, the lower are the BTM levels . During replacement therapy BTM levels increase .
Diabetes mellitus is a state of low bone turnover. Blood OC and CTX-I levels are low in type 1 and type 2 diabetes . PINP and TRACP5b levels are lower in type 2, but not type 1, diabetes. However, there are more data on BTMs in type 2 than type 1 diabetes. Bone ALP and NTX-I did not differ between diabetic patients and controls. As hyperglycemia does not interfere with BTM assays, these results do not seem to be due to measurement error. In type 2 diabetes mellitus, hyperglycemia, insulin resistance as well as higher secretion of sclerostin and osteoprotegerin may contribute to the low bone turnover . Observational studies on BTMs in type 2 diabetes mellitus should be interpreted cautiously because they do not account for the effects of antidiabetic drugs and vitamin D status. Mechanisms underlying low bone turnover in type 1 diabetes mellitus are not fully understood either.
65.3.4
Effect of drugs on bone turnover markers
Corticosteroids block bone formation dose-dependently. The fall in OC level is the most rapid (several hours) and followed by a milder decrease in PICP, PINP, and bone ALP . Bone resorption can rise after long treatment (>3 months) but data are inconsistent. Low dose prednisone (5 mg/day) and glucocorticoid replacement therapy decrease bone formation, not resorption . Inhaled corticosteroids decrease OC dose-dependently, slightly but significantly, whereas their effect on other BTMs is not significant .
The effect of corticosteroids is stronger after the menopause and depends on the disease and its severity . In bronchial asthma, BTMs reflect their undesirable effect on bone. Conversely, inflammatory diseases (e.g., rheumatoid arthritis) affect bone by themselves. In these patients, changes in the BTMs reflect mainly the pharmacologic effect of corticosteroids and depend on the severity of the disease at baseline .
Inhibitors of aromatase, used in the treatment of breast cancer, reduce residual estrogen secretion, which further accelerates bone turnover . In postmenopausal women with breast cancer, aromatase inhibitors increase the BTM levels by 10%–35% versus baseline . This increase can be prevented by bisphosphonates or tamoxifen .
In premenopausal women with endometriosis, gonadotropin-releasing hormone agonists block 17β-estradiol secretion and increase BTM levels (resorption, formation), which is followed by bone loss proportional to the increase in the bone turnover rate .
Antiepileptic drugs (AED) modify bone metabolism. Phenobarbital, carbamazepine, phenytoin, or oxcarbazepine stimulates hepatic cytochrome P450 hydroxylase increasing the synthesis of inactive 24-hydroxylated vitamin D forms . Patients receiving these AED have higher BTM levels . Nonenzyme-inducing AED (lamotrigine, valproic acid, topiramate) can increase serum OC, bone ALP, CTX-I, and ICTP without influencing vitamin D metabolism . However, their mechanism of action on bone is not known. Levetiracetam decreased serum CTX-I and had no effect on PINP . Discordant results may be due to methodological problems. Groups are small and composed of young patients. Treatment duration varies substantially. BTMs can be influenced by fasting status and season (vitamin D metabolism).
Statins (β-hydroxy-β-methylglutaryl-CoA reductase inhibitors) may influence bone turnover . However, in most studies, their effect on BTMs was weak or nonsignificant . The effect on BTMs did not differ between the lipophilic statins (atorvastatin, simvastatin) and hydrophilic statins (pravastatin, rosuvastatin).
Thiazolidinediones (TZD) are used in the treatment of diabetes. They induce the peroxisome proliferators–activated receptor in mesenchymal cells and promote their differentiation into adipocytes in preference to osteoblasts . Consequently, osteoblast number and activity decrease. TZD-induced changes in BTM levels vary markedly between the studies . Moreover, the effects of TZD on BTMs and BMD are disparate .
Vitamin K antagonists (VKA, oral anticoagulants) inhibit gammacarboxylation of OC and increase its undercarboxylated fraction . As OC gammacarboxylation is important for its secretion by osteoblasts, VKA-treated patients have lower levels of total OC . Other BTMs are not influenced by the oral anticoagulants .
Calcium and/or vitamin D supplements slightly reduce serum CTX-I and PINP in a dose and time dependent manner, which is mediated by suppression of PTH secretion . This effect depends on the doses of vitamin D and/or calcium, schedule of the trial (daily low dose, single high dose of vitamin D), season, comorbidities, and duration of the study. The effect is greater in vitamin D–deficient subjects, especially in the elderly or in malnourished individuals.
These effects vary by the factor, its intensity, and the BTM. Circadian rhythm is more pronounced in case of CTX-I compared to other BTMs. The circannual rhythm of BTM levels is more pronounced in the elderly than young persons. The effect of the fasting/eating pattern on BTMs is stronger than that of the physical activity. The effect of diseases on the BTMs varies by their severity. Drugs vary by their effect which is stronger for inhibitors of aromatase and weaker for TZD or AED. Their effect on the BTMs depends on the dose, duration of treatment, and underlying disease.
65.4
Preanalytical variability in clinical practice
As the prenalytical factors influence BTMs, they should be assessed to interpret BTM results correctly . Several factors may coexist in one person. In patients with rheumatoid arthritis, BTMs may depend on the disease, corticosteroid therapy, and immobility. In HIV-infected subjects, high BTM levels may be due to the lifestyle, effect of the virus, inflammation, and therapy. Home-bound persons have high BTM levels due to poor mobility, malnourishment, and low sunlight exposure. Some factors influence one but not other BTMs, for example, inhaled corticosteroids and VKA decrease OC. The results have to be assessed cautiously, for example, in women treated with aromatase inhibitors, a rise in the BTMs may obscure the development of bone metastases.
Biological samples should be collected under standardized conditions, preferably in the fasting state in the morning. In clinical practice, fasting morning sample is necessary for CTX-I, but not for PINP. The 24-hour urinary collection is representative of the overall bone metabolism but difficult to perform in a controlled way. Second morning void [(SMV) spot] may be collected easily and repeatedly in a controlled way. Most of the reference data for the urinary markers come from the SMV urines. The correlation between the SMV and 24-hour urinary results varies according to the marker and the cohort . The 24-hour BTM excretion not corrected for creatinine is artifactually underestimated when renal function is not in a steady state. The BTM excretion per mg of urinary creatinine is artifactually overestimated in cases of low creatinine excretion in sarcopenia. The BTM amount per glomerular filtrate volume assumes that the glomerular filtration is the same as that of creatinine and that there is no tubular reuptake.
65.5
Clinical use of bone markers in postmenopausal osteoporosis
65.5.1
Bone markers and rate of bone loss
Bone turnover increases rapidly after the menopause . In postmenopausal women, BTM levels correlate negatively with BMD at each skeletal site, and this correlation strengthens with age . After the menopause the BTMs increase and bone loss becomes rapid . High BTM levels are associated with subsequent bone loss in peri- and early postmenopausal women . This link weakens with age and, in elderly women, is weak or nonsignificant . Although significant, the association between BTMs and bone loss varies by BTM and skeletal site with a large overlap of rates of bone loss between women with normal and high BTM levels. The utility for BTMs to predict the accelerated bone loss is modest . Indeed, the bone loss during 3 years (3%–4%) is similar to the precision error of repeated measures in a single subject and it is difficult to obtain a valid estimate of the individual bone loss in a short follow-up. These studies used older BTM assays with CV of 10%. Thus the measured values of a BTM in one sample could vary by >30% .
This bias can be reduced by frequent measurements of BMD and BTM levels, longer follow-up and/or statistical correction for the error of assessment of the BTMs and of the rate of bone loss. When distal radius BMD was measured every 3 months over 24 months, the correlation coefficients between the rate of bone loss and the BTM levels were 0.7–0.8, that is, higher than those obtained using yearly BMD measurements (0.2–0.4) .
Thus postmenopausal bone loss is driven by rapid bone turnover. Its inhibition by antiresorptive drugs can maintain BMD. However, the association between BTM levels and rate of bone loss is poor partly due to the high errors of assessment of both. BTM cannot be used for the prediction of rapid bone loss at the individual level.
65.5.2
Bone turnover markers in the assessment of fracture risk
Detection of women at high risk of fracture is a difficult task in clinical practice. Only 40% of fractures occur in women with BMD T -score<−2.5 . Other measures of fracture risk are needed. Retrospective studies show high bone resorption markers in patients with prior fracture . However, these results are influenced by many factors: time elapsed between the fracture occurrence and biological sampling, acute changes in body fluid, changes in hormone levels leading to a catabolic stress, for example, high cortisol secretion after a trauma may lower OC level.
Data on the predictive value of bone formation markers are mostly negative. High bone ALP level was associated with a high fracture risk in some , but not all , studies. The associations between PINP and fracture risk were inconsistent in the models that were not-adjusted for BMD and became nonsignificant after adjustment for BMD .
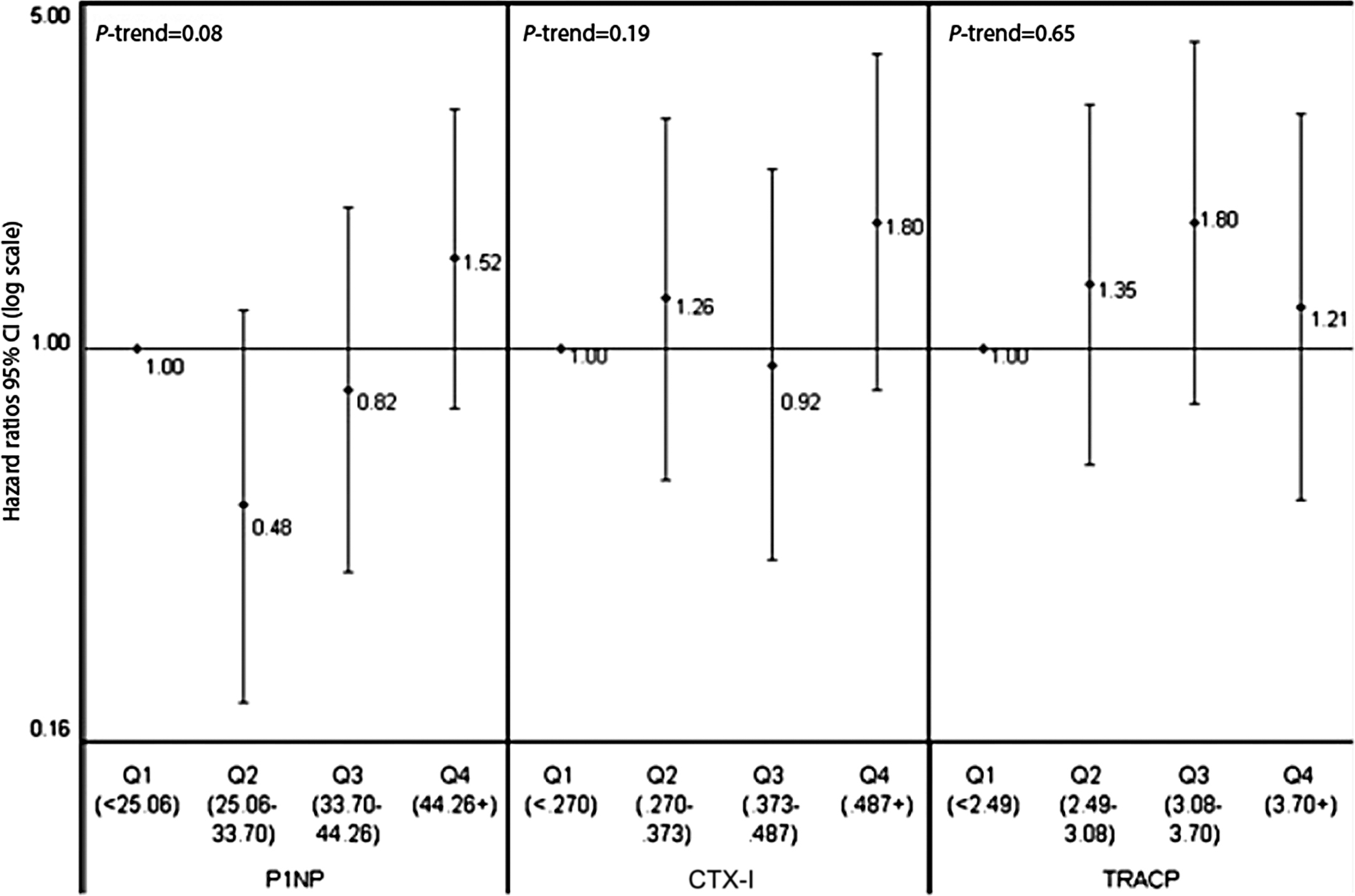
Data on the link between bone resorption markers and fracture risk show different trends for serum markers and for urinary markers. In most of the prospective follow-ups of less than 10 years (cohort, case–control), higher levels of urinary bone resorption markers predicted fragility fracture, mainly major osteoporotic or multiple fractures, often also when adjusted for BMD . By contrast, data on the link between blood CTX-I and fracture risk are inconsistent, regardless of the fracture type and regardless of the statistical model (adjusted for BMD or not) ( Fig. 65.4 ).
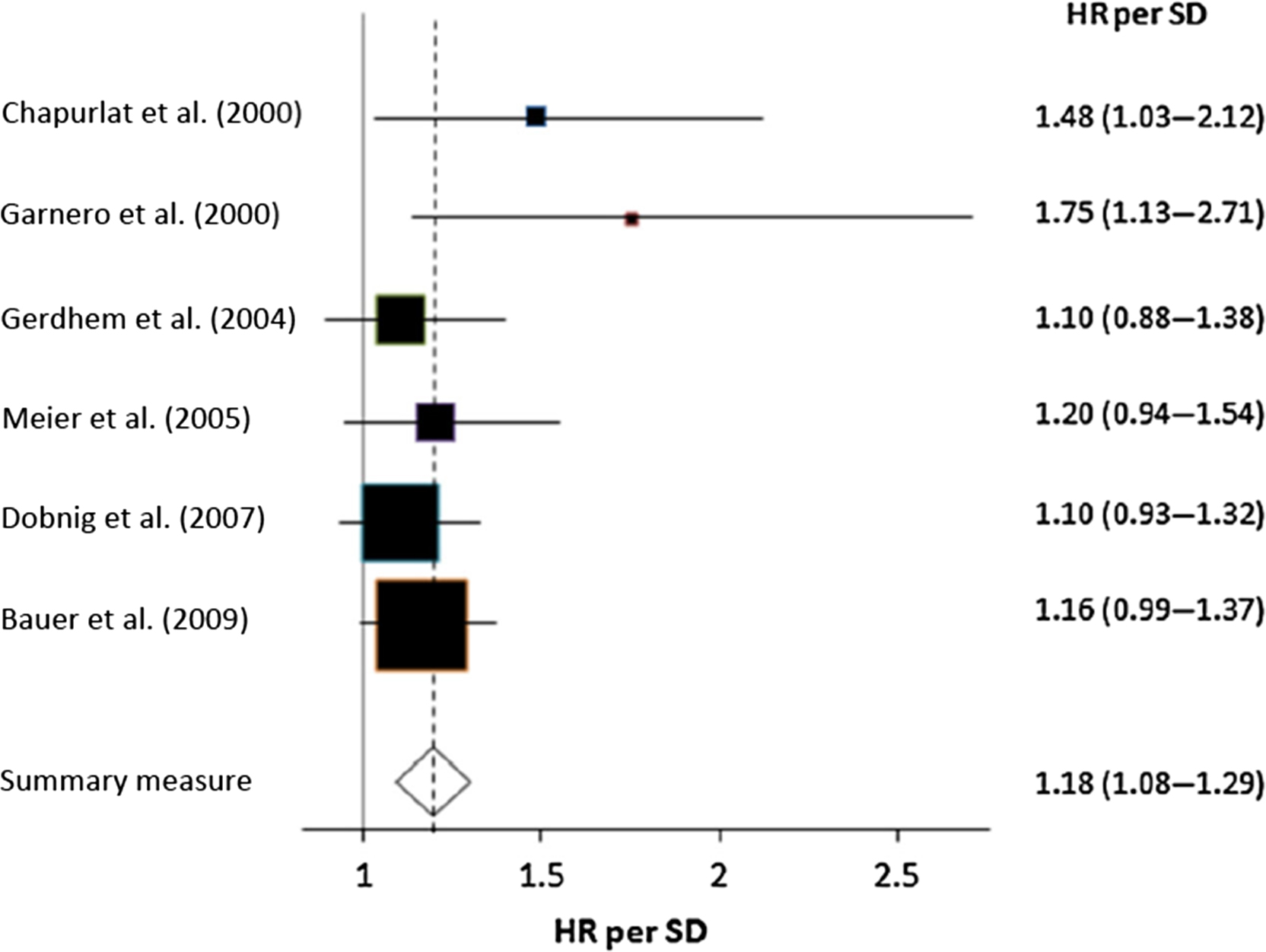
Urinary BTMs may predict fractures because their higher levels reflect higher bone resorption and lower muscle mass which is associated with disability (creatinine in the denominator). Data on the link between sarcopenia and fracture risk are inconsistent ; however, the associations between urinary markers and hip fracture risk became nonsignificant after adjustment for disability . The interpretation of results should account for the number of fractures in the investigated cohort, standardized sample collection, and follow-up duration. BTMs predicted fractures in short-term follow-ups (≤5 years), but not in the long-term ones .
The positive link between BTMs and fracture risk suggests that high bone turnover is a determinant of bone fragility . High bone turnover is associated with a faster bone loss which is a risk factor for fracture regardless of BMD . High BTMs are associated with higher activation frequency . This may lead to the loss of trabeculae which compromises bone strength but is not detected by BMD. In women with high BTMs, bone may be resorbed again before it has attained normal mineralization and normal degree of posttranslational modifications of proteins, two factors that determine bone strength . Older women with hip fracture had higher urinary α/β-CTX-I ratio showing lower β-isomerization . High α/β-CTX-I ratio predicted fractures regardless of BMD . Thus poor bone matrix composition may compromise bone strength. Therefore the link between BTMs and fracture risk may be found only for high BTM levels. Thus cohorts composed of home-dwelling volunteers may include few subjects with very high BTM levels.
Few data concern the use of BTMs for fracture prediction in clinical practice. It is not clear whether BTMs may be used as a sole indicator of the fracture risk in the institutionalized elderly for whom BMD measurement by dual-energy X-ray absorptiometry (DXA) with the necessary transport would be unpractical.
65.5.3
Other metabolic markers of bone fragility
In three cohort studies, impaired OC gammacarboxylation was associated with a higher risk of fracture, mainly hip fracture . Impaired OC gammacarboxylation may reflect vitamin K deficiency or be a nonspecific by-stander of the nutritional deficits leading to frailty, rapid bone loss, and high risk of fall.
Lower serum levels of insulin-like growth factor I was associated with a higher risk of fracture independently of BMD in older men and women . However, data are inconsistent and vary according to the fracture type.
Homocysteine (Hcy) appeared to be a fracture risk predictor. A high level was associated with higher fracture risk (mainly hip fracture) mainly in the frail elderly . Hcy stimulates osteoclasts directly and inhibits lysyl oxidase, which is involved in the extracellular synthesis of PYD and DPD . Hcy-induced decrease in collagen cross-linking may compromise bone mineralization and reduce bone strength . However, the results of clinical studies are inconsistent. Therefore Hcy should not be used for fracture prediction. Moreover, Hcy-lowering treatment did not decrease fracture risk .
Pentosidine, an advanced glycation end product (AGE), is generated by nonenzymatic glycation of amino groups. It consists of lysine, arginine, and ribose and forms crosslinks between two collagen molecules. In Japanese subjects, high pentosidine levels (serum, urine) predicted vertebral fracture after adjustment for BMD . In Caucasian subjects, high urinary pentosidine levels were associated with higher vertebral fracture risk only in diabetic individuals but not in population-based cohorts . AGEs accumulate in bone and other tissues. Their accumulation increases with age, hyperglycemia, oxidative stress, and poor renal function . Thus high pentosidine level may reflect poor health status. Higher bone pentosidine content was associated not only with low bone strength but also with lower bone resorption . Overall, the inconsistent clinical and experimental findings have resulted in a progressive decrease in interest in pentosidine as a BTM.
Cystatin-C is filtered in glomeruli and metabolized in the proximal tubular cells. Its serum level negatively correlates with the glomerular filtration rate and so it is a marker of chronic kidney disease. In several cohorts, high serum cystatin-C level was associated with a higher risk of hip and nonspine fracture after adjustment for confounders . The link persisted after adjustment for hip BMD . These data are in line with the high fracture risk in renal failure . However, cystatin-C reflects renal function and not the bone turnover rate.
Inflammatory cytokines induce loss of bone and muscle. Thus their high activity, measured directly or indirectly by serum C-reactive protein (CRP), may reflect high fracture risk. After adjustment for BMD, high CRP was associated with higher fracture risk (mainly vertebra and hip) in some , but not all , studies. Higher fracture risk was found in subjects with higher levels of certain inflammatory cytokines, for example, interleukin-6 (IL6), IL6 soluble receptor (IL6-SR), tumor necrosis factor alpha (TNFα), and TNFα-SR . However, data are inconsistent and vary according to the cytokines, fracture site, cohort, and statistical model. Fracture risk was elevated mainly in subjects who had elevated levels of several cytokines simultaneously and in the models that were not-adjusted for BMD . Therefore the use of inflammatory cytokines for fracture prediction is not recommended.
65.5.4
Bone turnover markers for the assessment of the treatment efficacy
BTMs reflect the metabolic effect of drugs on bone turnover, help to establish the lowest dose of the drug inducing the required change in BMD, and are helpful in the bridging studies. BTMs can be measured repeatedly and changes in their levels are larger than those in the histomorphometric parameters. Changes in BTM levels are also correlated with the treatment-related increases in BMD and reduction in fracture risk. BTMs are useful for profiling the mode of action of a drug, such as the “anabolic window” induced by anabolic drugs or “uncoupling” induced by romosozumab.
A challenge in treating osteoporosis is that the patient does not feel any subjective improvement during the treatment. The use of DXA-measured BMD as a surrogate of the treatment efficacy has its limitations (high precision error, long time to obtain a sufficient gain in BMD allowing to detect if a patient is responding to therapy, small part of the antifracture efficacy is explained by the change in BMD) . The lack of subjective improvement and of surrogate markers to detect the therapeutic effect may contribute to the poor adherence and persistence to treatment. Recent data suggest that, during antiosteoporotic therapy, BTMs may provide an early surrogate of treatment efficacy .
BTMs also present limitations. Preanalytical and analytical variability have been mentioned. They make no distinction between the bone resorption and the further catabolism of the degradation products of bone collagen. Antiresorptive drugs can have different effects on the bone collagen degrading enzymes resulting in variable changes in different bone resorption markers . A part of urinary fDPD is produced in the renal tubular cells . It depends on the mass of DPD-containing peptides released from bone and on renal function. BTMs make no distinction between the cortical and trabecular bone; however, drugs can have different effects in both compartments. The association between the changes in BMD, BTMs and the fracture risk is not fully understood, for example, alendronate decreases BTM levels more than risedronate and raloxifene decreases BTM levels less than risedronate, whereas these three drugs decrease the vertebral fracture risk to a similar extent .
65.5.4.1
Metabolic effect of antiosteoporotic treatments
Inhibition of bone resorption by antiresorptive drugs at clinically relevant doses leads to a decrease in the levels of bone resorption markers followed by a plateau. This decrease occurs after 3 months for oral bisphosphonates and selective estrogen receptor modulators (SERMs), after 1 month for i.v. bisphosphonates and after 1 week for denosumab . As bone formation continues in BMUs activated before treatment, bone formation markers are stable for several weeks. They decrease when osteoblasts fill in the lower number of BMUs formed during the treatment, then they plateau. This sequence reflects the physiological coupling of bone formation and resorption. BMD rapidly increases during the early period, when bone resorption decreases and bone formation continues at the previous rate. Some drugs maintain osteoclast presence and the cross-talk between osteoclasts and osteoblasts. Bisphosphonates may inhibit apoptosis of osteoblasts and osteocytes and stimulate osteogenic differentiation of mesenchymal stem cells . However, it is not known if this phenomenon may influence serum levels of bone formation markers.
Changes in the BTMs during antiresorptive therapy depend on the mechanism of action of the drug, degree of inhibition of resorption and route of administration. Bisphosphonates and calcitonin inhibit the activity of mature osteoclasts, denosumab (anti-RANKL antibody) inhibits osteoclastogenesis and the activity of mature osteoclasts, and catK inhibitors bind to the enzyme and block its activity . Subcutaneous denosumab inhibited bone resorption 12 hours after injection and i.v. ibandronate decreased bone resorption 2 days after administration ( Fig. 65.5 ). Intravenous bisphosphonates decrease bone resorption faster than oral ones . In contrast, various regimens of i.v. ibandronate induced similar temporal sequences of the changes in BTM levels .
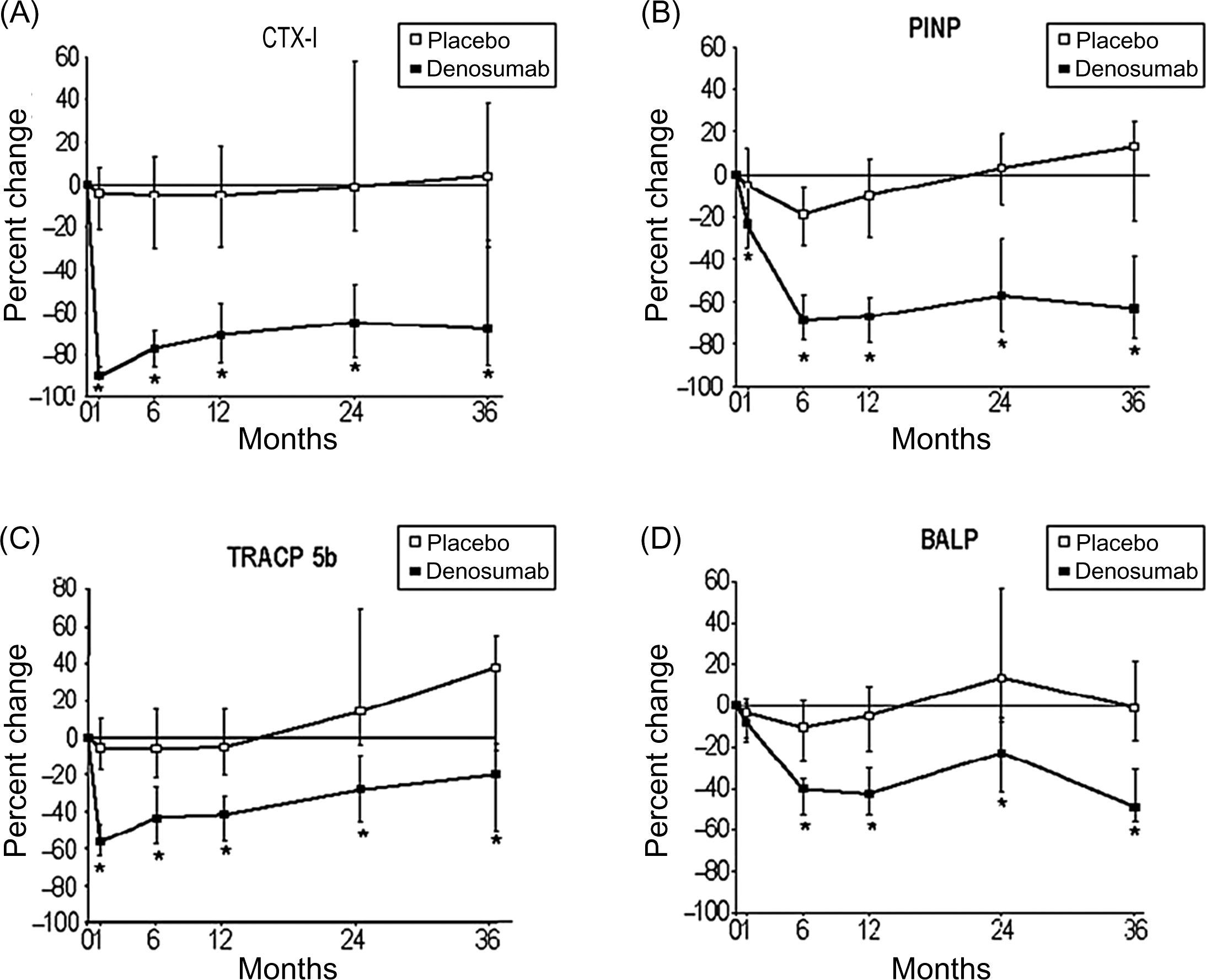
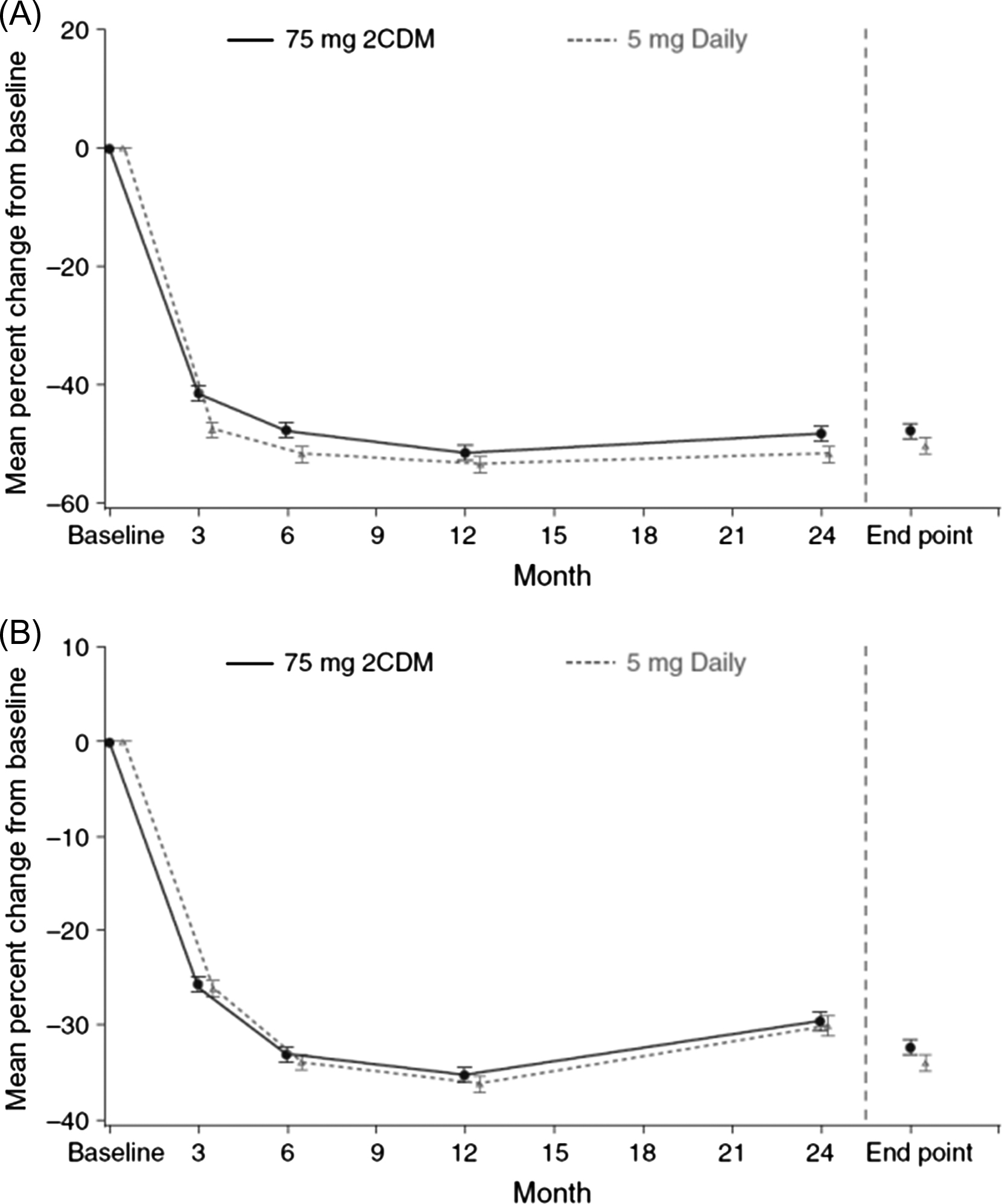
Bisphosphonates inhibit bone turnover and decrease the BTM levels to a greater degree than SERMs . For bone formation markers the order of magnitude is similar but the reduction is smaller. The reduction in the BTM levels is dose-dependent. For alendronate the pattern of the decrease in BTM levels is similar for daily and weekly doses . Also for risedronate, the pattern of the decrease in BTMs did not differ for daily, weekly, and monthly dosing (150 mg once a month or 75 mg on 2 consecutive days a month) ( Fig. 65.6 ).
Despite somewhat disappointing results, clinical studies on the CatK inhibitors provided data on the changes in the BTM levels during the antiresorptive therapy. CatK inhibitors block the catabolism of type I collagen . Collagen is first degraded by MMP, which releases ICTP. Then, CatK degrades ICTP to release CTX-I and releases NTX-I at the N-terminal end. As CTX-I and NTX-I are degraded directly by CatK, their decrease may overestimate the antiresorptive effect. This is in line with the greater drop in the CTX-I and NTX-I compared with a milder decrease in total DPD excretion . By contrast, the activated osteoclasts resorb the superficial layer of bone, but the released fragments cannot be catabolized by CatK . Therefore serum ICTP increases ( Fig. 65.7 ).
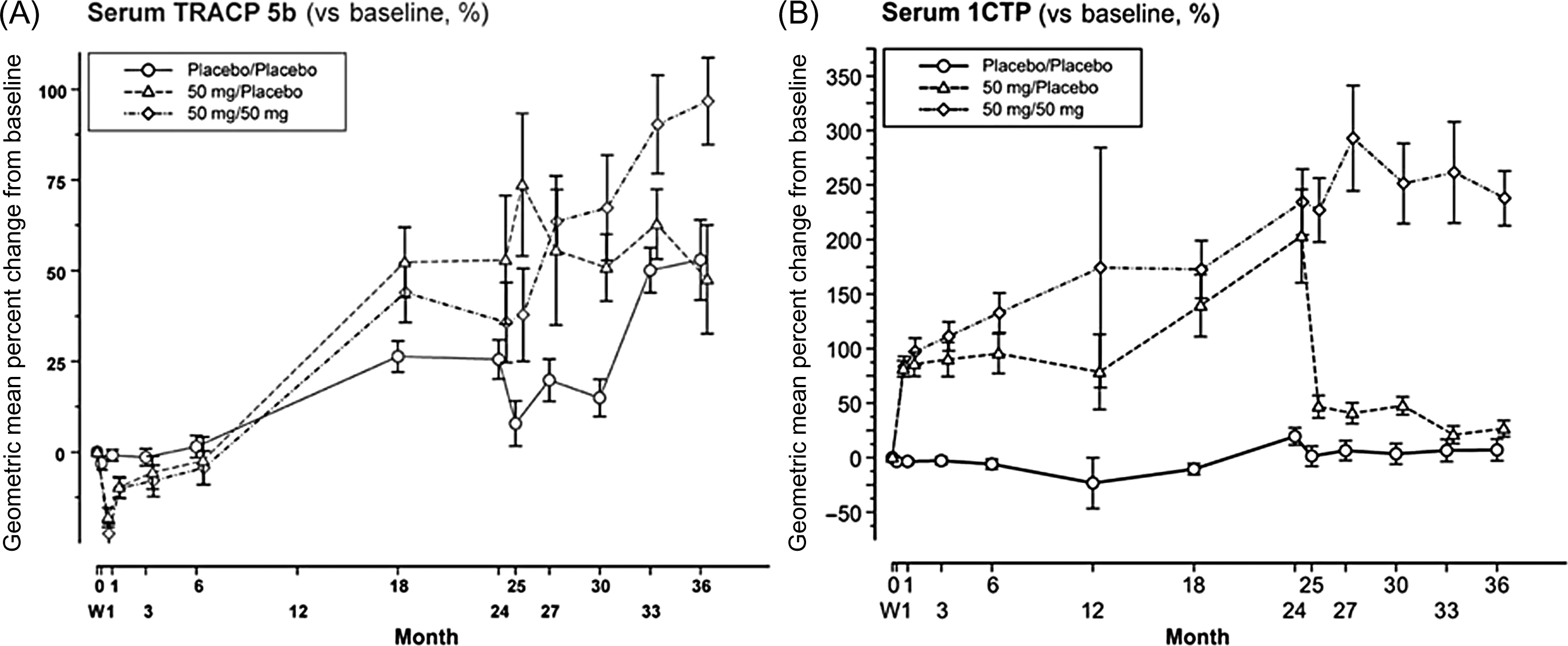
CatK inhibitors decrease the activity, but not the number of osteoclasts. As TRACP5b level reflects the number of osteoclasts, its level is stable or increases during the treatment. As the osteoclasts stimulate osteoblast activity, bone formation decreases less in patients treated with CatK inhibitors compared with patients treated with bisphosphonates . The imbalance between strongly inhibited bone resorption and slightly lower bone formation results in the increase in BMD . However, after the initial decrease, BTMs slightly increase despite treatment, which may explain the slowdown of the BMD increase . Therefore ICTP and TRACP5 do not reflect the suppression of bone resorption by CatK inhibitors.
Bone formation–stimulating drugs (recombinant human PTH(1-34), i.e., teriparatide or PTH(1-84) increase bone formation rapidly ( Fig. 65.8 ). PINP increases 3 days after the beginning of treatment and is followed by other BTMs . The possible explanation is that PTH stimulates early osteoblastic cells that express type I collagen, but not yet bone ALP or OC . The bone formation markers peak after 1–6 months then level off or decrease but remain above the baseline values. In this early phase of treatment, bone resorption remains low. Then, bone resorption slowly increases, but less so than bone formation . The gap between the rapidly increasing bone formation and slowly increasing bone resorption generates the “anabolic window” characterized by a rapid increase in BMD . Therefore the PTH-induced bone gain is determined by a positive balance at the level of a BMU with an excess of formation over resorption.
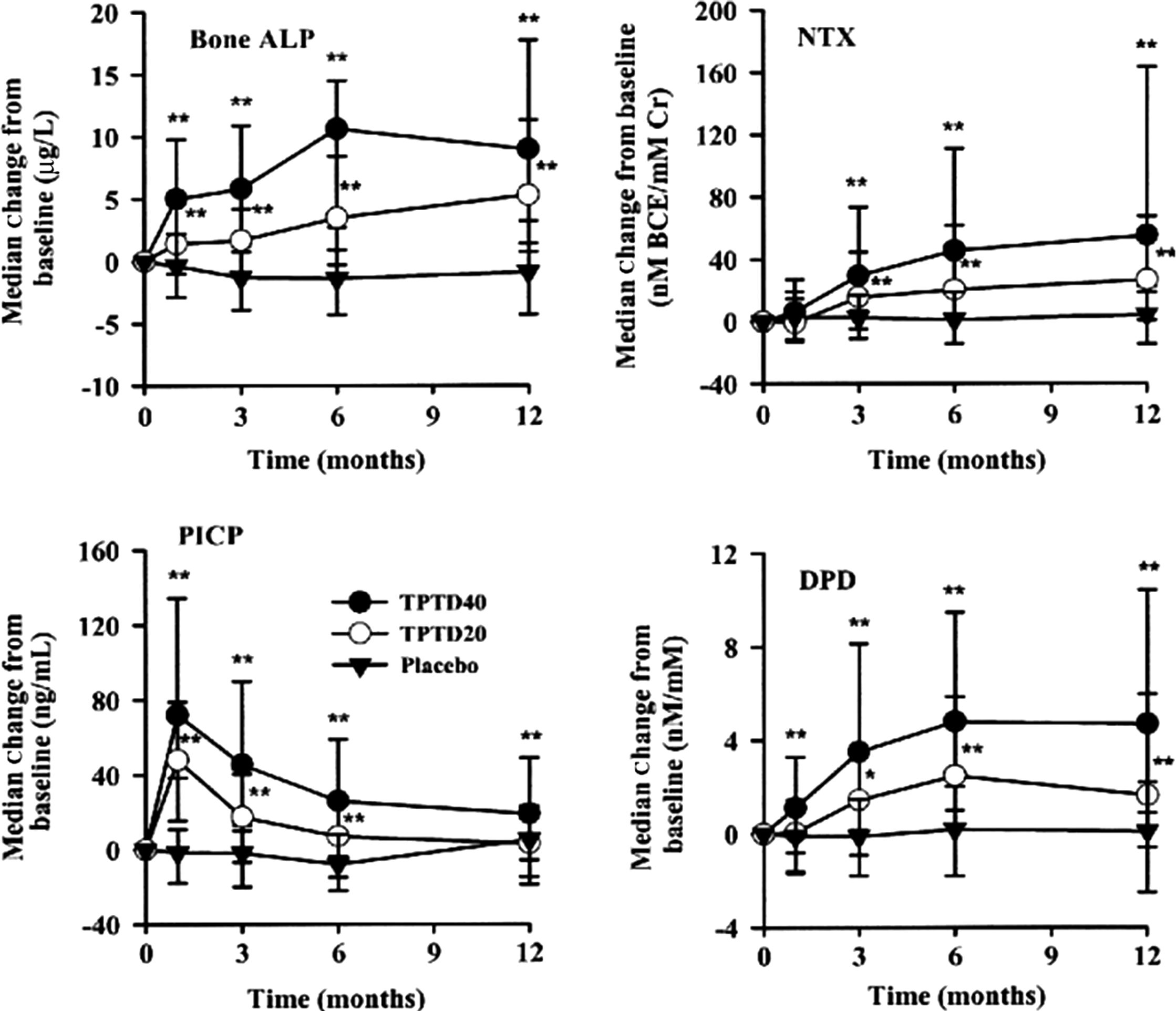
Abaloparatide, another anabolic drug, is a synthetic peptide analog of PTH-related protein. In osteoporotic women, it stimulates bone formation and resorption rapidly and dose-dependently, less than does teriparatide, but the increase in formation relative to resorption is the same . Similar to teriparatide, BTMs decrease after 12–18 months of therapy, whereas BMD continues to rise and attains higher BMD versus teriparatide.
Romosozumab is also an anabolic drug, consisting of a monoclonal antibody that blocks sclerostin, which is an inhibitor of bone formation . It induces a substantial (150%) and rapid, but transient, increase in PINP level (peak after 14 days) and a decrease in serum CTX-I (about 50%) . The rapid increase in PINP is followed by a slower increase in serum OC and bone ALP. This dissociation in bone turnover in favor of bone formation is associated with a rapid increase in BMD. In postmenopausal women with low BMD, PINP returns to baseline within 6 months, whereas CTX-I remains low and stable.
Strontium ranelate increases bone ALP and lowers serum CTX-I . Then, both plateau suggesting a dissociation between resorption and formation. However, data are not consistent (e.g., PINP decreases), and this dual effect is not found by bone histomorphometry .
65.5.4.2
Bone turnover markers and dose-finding studies
BTMs are used for the assessment of therapeutic equivalence of various doses or regimens of the same drug. They help to establish their optimal dose because the changes in BTMs are more rapid compared with BMD.
Higher doses of antiresorptive agents induce faster and greater decreases in BTM levels with a lower steady state and greater rise in BMD. During treatment with SERMs or oral bisphosphonates the decrease in BTMs is dose-dependent and statistically significant after 1 month for bone resorption and after 6 weeks for bone formation . BTMs attain their lowest levels (after 3 months for bone resorption and 6 months for bone formation) and then remain stable. Such trends are found for SERMs, alendronate, risedronate, and oral ibandronate. The trend for an oral CatK inhibitor was similar but more rapid. Urinary bone resorption markers decreased by 60%–70% the day after the administration of the first dose and bone formation markers decreased by 20%–30% after 1 month of treatment .
Intravenous bisphosphonates and subcutaneous denosumab rapidly decrease bone resorption . The effect is dose-dependent for bisphosphonates but not always for denosumab. The duration of the low bone resorption and its residual depression before the next injection are dose-dependent for both. Bone formation decreases later, progressively and dose-dependently. The steady state is attained after 3 months for denosumab and after 6 months for bisphosphonates. The higher the dose of the drug, the lower the bone resorption level before the next injection of the drug.
The anabolic agent, PTH , induces dose-dependent increases in BTM levels, mainly bone formation markers, although the changes were lower than with teriparatide . Romosozumab rapidly increased bone formation . The increase in bone formation and the duration of the effect are dose-dependent, which permits the establishment of the optimal dose and the optimal interval between the injections.
In phase II studies BTMs help to define the right dose. However, i.v. bisphosphonates, catK inhibitors denosumab, and romosozumab exert a rapid effect . Their measurement in phase I studies may provide a useful hint on the clinically relevant doses.
65.5.4.3
Bone turnover marker levels and therapeutic efficacy of antiosteoporotic treatments
The decrease in BTM levels during the first months of antiresorptive treatment is correlated with the long-term increase in BMD . The early decrease in the BTM level exceeding a predefined threshold predicted the “positive response” (long-term increase in BMD above the least significant change, i.e., 2.8 times the precision error of BMD measurement). The threshold was fixed for various BTMs and various drugs . However, the increase in BMD is poorly correlated with the decrease in fracture incidence . For any change in BMD (vs baseline), fracture risk is lower in the treated than in the placebo group .
In some , but not all , studies, women with higher initial BTM levels had a slightly greater reduction in the fracture incidence during treatment. Data vary by the BTM, initial BMD, and fracture site. The effect is stronger for lower BMD. As women with high BTMs have higher fracture incidence, a similar relative fracture risk reduction corresponds to the higher absolute reduction, that is, higher number of avoided fractures .
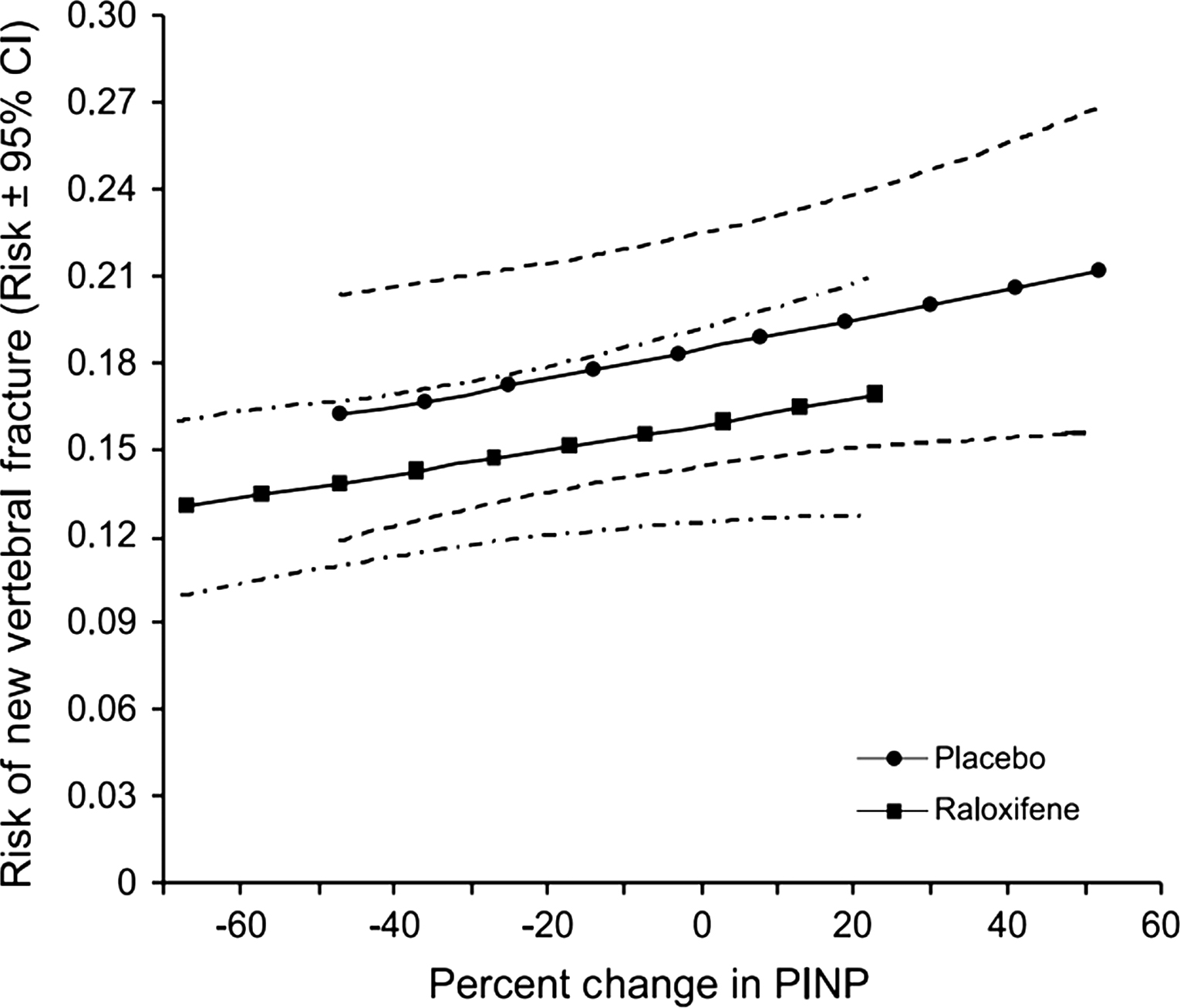
A greater decrease in BTMs during the first year of antiresorptive treatment is associated with a greater antifracture efficacy over 3 years. Such a link was found in women treated with alendronate, risedronate, and raloxifene . In various studies, different markers were found as those that predicted the reduction in fracture risk the best, it could be bone ALP, PINP or another marker. The vertebral fracture incidence was similar in the active treatment and placebo groups for a given decrease in BTM levels and for a given on-treatment BTM level. Women with the greatest decrease in the BTM levels had the lowest fracture incidence, whereas in women who had stable BTM, fracture incidence was similar to that in the placebo group ( Fig. 65.9 ).
In patients treated with oral bisphosphonates a greater decrease in the BTM levels was also associated with a higher reduction of the incidence of nonvertebral fractures. This association was found in patients treated with alendronate (bone ALP) and in patients treated with risedronate (urinary NTX-I, serum CTX-I) .
The association between the decrease in BTM levels and antifracture efficacy was stronger for bone formation markers (bone ALP, PINP) versus bone resorption markers, for vertebral fractures (vs nonvertebral or hip fractures), and stronger for individuals with low BMD (vs those with higher BMD) . Vertebral fractures and fractures in patients with low BMD may depend more on the intrinsic bone fragility partly related to the bone turnover rate. By contrast, fractures in women with higher BMD and nonvertebral fractures may depend more on the frequency and severity of traumas (e.g., fall propensity). The stronger association with bone formation markers may be related to the purely technical factors, such as better reproducibility, lower circadian variability.
Data on the link between BTM levels and antifracture efficacy during intermittent treatment are scanty. During intravenous and oral intermittent antiresorptive therapy the levels of bone resorption markers are very low soon after the drug administration and slightly increase before the administration of the next dose . For various regimens of ibandronate the extent of reduction of bone resorption at the end of the interval determines the antifracture efficacy of the intermittent antiresorptive treatment . In patients treated with zoledronate, a greater 12-month decrease in PINP level was associated with a lower risk fracture over 3 years .
Thus the fracture risk depends largely on the bone turnover rate and its change. However, various drugs from the same group may induce similar reduction in fracture risk despite variable changes in BTM levels. Alendronate decreases BTM levels more than risedronate but both reduce the fracture incidence to a similar extent .
The early teriparatide-induced rise in BTMs and their levels during the early phase of treatment (1 or 6 months) correlated positively with the subsequent increase in BMD, mainly trabecular volumetric BMD (vBMD) . PINP may be useful for monitoring teriparatide therapy, as the increase in PINP level by >10 pg/mL predicts a greater increase in BMD . Teriparatide-induced changes in BTMs were not associated with fracture risk. However, the link between changes in BMD, BTMs, and bone formation assessed by histomorphometry is not clear. After 1 month, PINP increases by 65%, whereas bone formation assessed by histomorphometry increases by factors of 4–5 according to the bone envelope . PINP increases for 3–6 months and then decreases. Other BTMs are elevated for 12–18 months then decrease despite a continuous increase in BMD.
65.5.4.4
Bone turnover marker levels after discontinuation of antiosteoporotic treatments
Bisphosphonates are accumulated in bone and not metabolized . After their withdrawal, they are released from the bone slowly, presumably due to bone remodeling. Thus osteoclasts may be inhibited by the drug released from the bone matrix.
Upon withdrawal of alendronate after 5–7 years of therapy, BTM levels slowly increased but did not return to baseline . This increase was followed by a decrease in BMD and could be associated with a higher fracture risk . In a randomized comparison of alendronate, risedronate, and ibandronate all given for 2 years and then stopped, there was only partial resolution of suppression of BTMs over a further 2 years . The greater the resolution, the greater was the rate of bone loss . This was not found in all studies nor was the BTM change related to fracture; however, the studies were too small to evaluate this .
Urinary NTX-I increased after withdrawal of risedronate promptly and to a similar extent in women treated for 2 years and in those treated for 7 years . The initial increase in BTM levels is faster after the discontinuation of risedronate compared to alendronate . Withdrawal of zoledronate after 3 years of treatment resulted in a slight increase in serum PINP, but even 4 years after the last zoledronate dose, the PINP level was 50% below the baseline level . The rate of resolution of PINP was slower after stopping zoledronate as compared to alendronate .
The rate of increase in BTM levels and of bone loss after withdrawal of various bisphosphonates may reflect the difference in the degree of inhibition of bone turnover or the difference in the degree of their accumulation in bone matrix, which depends on the duration of treatment and on the affinity of the drug to bone mineral. The lower the cumulative dose, the faster the BTMs increase.
HRT, SERMs, denosumab, and CatK inhibitors do not accumulate in bone. Their withdrawal is followed by a rapid increase in the BTMs to the values exceeding pretreatment levels ( Fig. 65.10 ). The increase in BTMs after withdrawal is reversible by retreatment. Retreatment with denosumab 12 months after its withdrawal decreased BTM levels and increased BMD to values similar to those in patients receiving continuous treatment . Of note, discontinuation of denosumab is followed by rapid bone loss and an increase in multiple vertebral fracture risk .
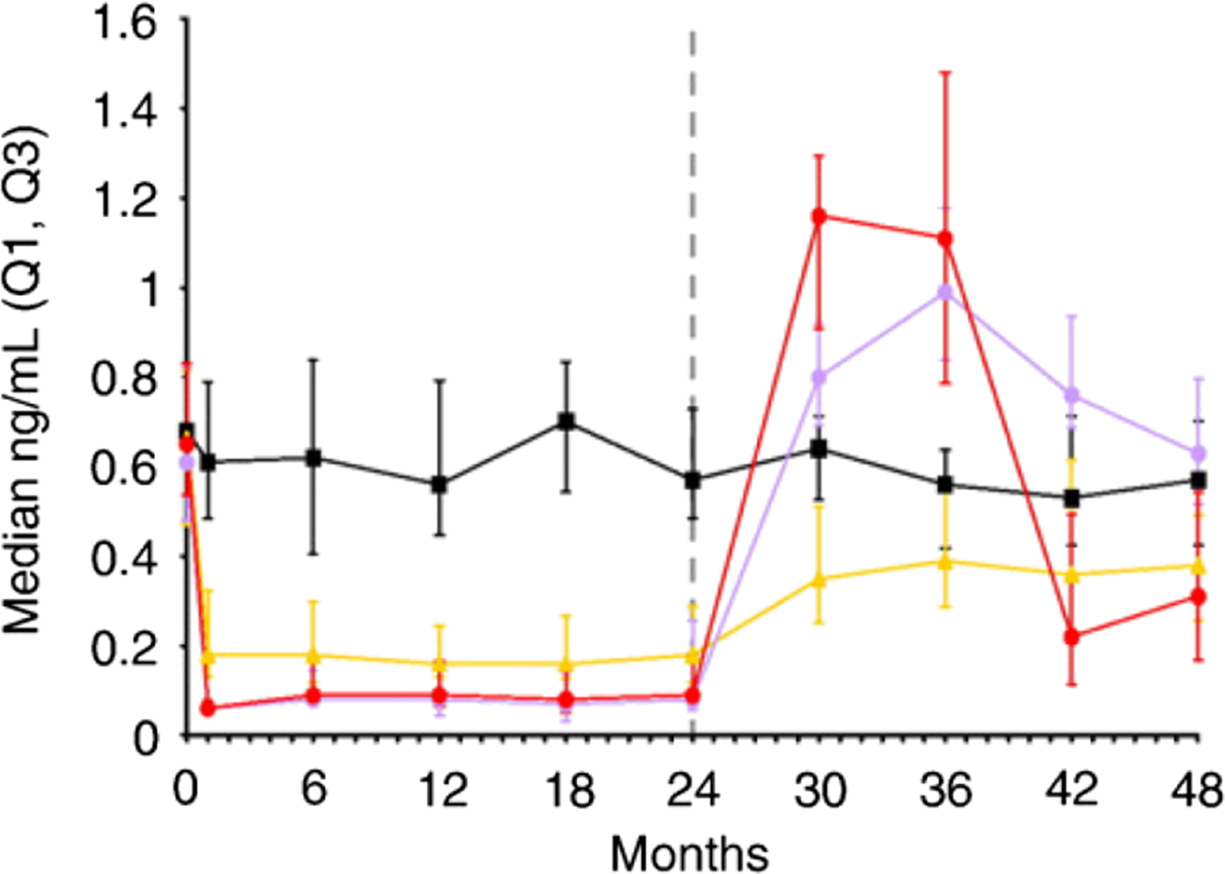
Withdrawal of PTH after 1 year of treatment was followed by a return of BTM levels to baseline and a decrease in trabecular vBMD . After withdrawal of teriparatide, BTMs (PINP, OC, urinary NTX-I) decreased and returned to baseline, which was associated with a decrease in BMD at the lumbar spine and hip . The retreatment with the same agent increased BTM levels; however, the increase was much lower compared with the initial treatment in treatment-naïve subjects .
After romosozumab withdrawal a rapid increase in serum CTX-I was followed by a milder increase in PINP and a decrease in BMD at the lumbar spine and total hip ( Fig. 65.11 ) .
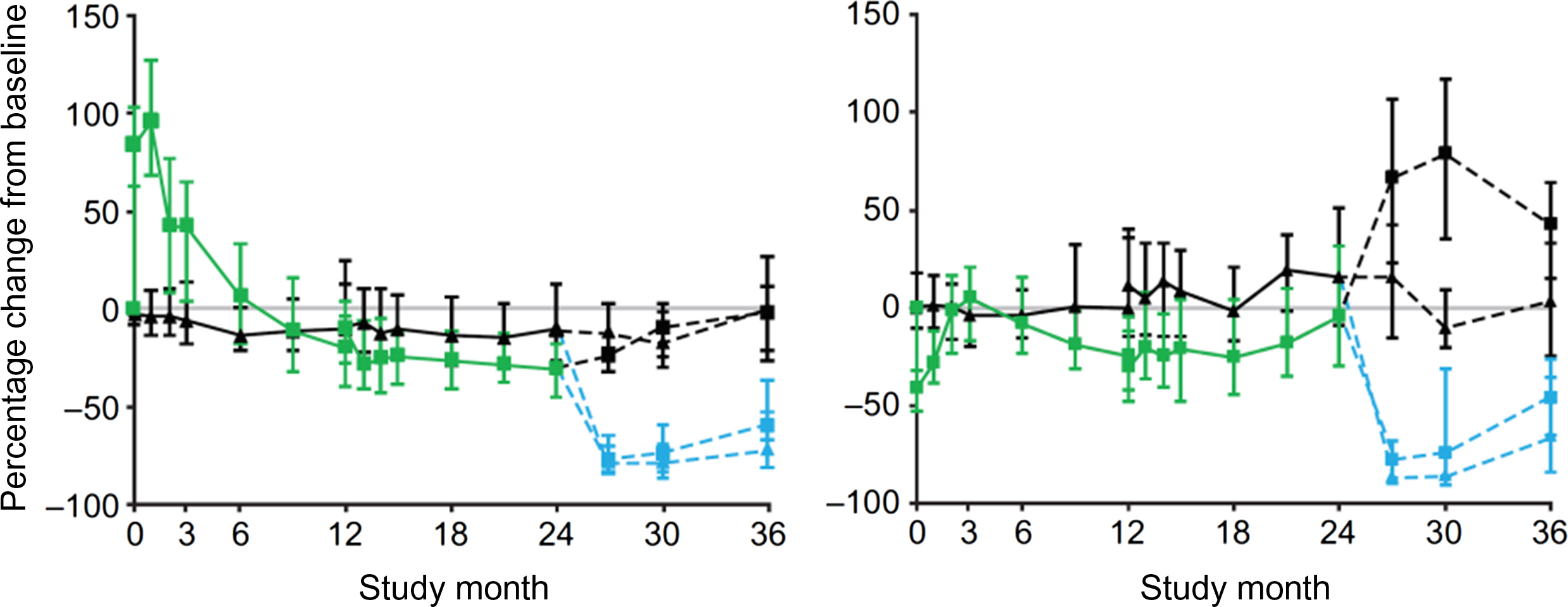
After discontinuation of strontium ranelate administered for 4 years, bone ALP decreased whereas serum CTX-I increased .
Conclusions on the changes in BTM levels in long-term studies should be considered with caution. Comparisons between drugs in the same class group are limited by different duration of treatment in various protocols. The analyses are performed in small groups of participants. The data obtained for BTMs and BMD cannot be extrapolated to the fracture risk. It is not clear which of the following measurements predict the loss of antifracture efficacy: relative change in BTM level, absolute change, BTM level after a predefined time since the last dose of drug.
65.5.4.5
Combination therapy and bone turnover markers
Several combinations of antiosteoporotic drugs were assessed in women. Alendronate and PTH administered jointly decreased bone resorption (serum CTX-I), but less so than alendronate alone . The combination increased bone formation, but less than PTH alone. Combination therapy did not provide greater change in BMD than either drug alone. These data do not show any evidence of synergy between these agents.
Zoledronate and teriparatide administered jointly decreased serum CTX-I similarly to zoledronate alone . After 1 year CTX-I increased and exceeded the baseline values. In the combination therapy group, PINP remained lower than in the teriparatide group.
In osteoporotic women the combination of teriparatide and raloxifene increased bone formation similarly to teriparatide alone . In this group, bone resorption increased less and hip BMD increased more versus teriparatide alone ( Fig. 65.12 ). Thus in the case of combination of teriparatide with a weaker resorption inhibitor, the effect was dominated by teriparatide.
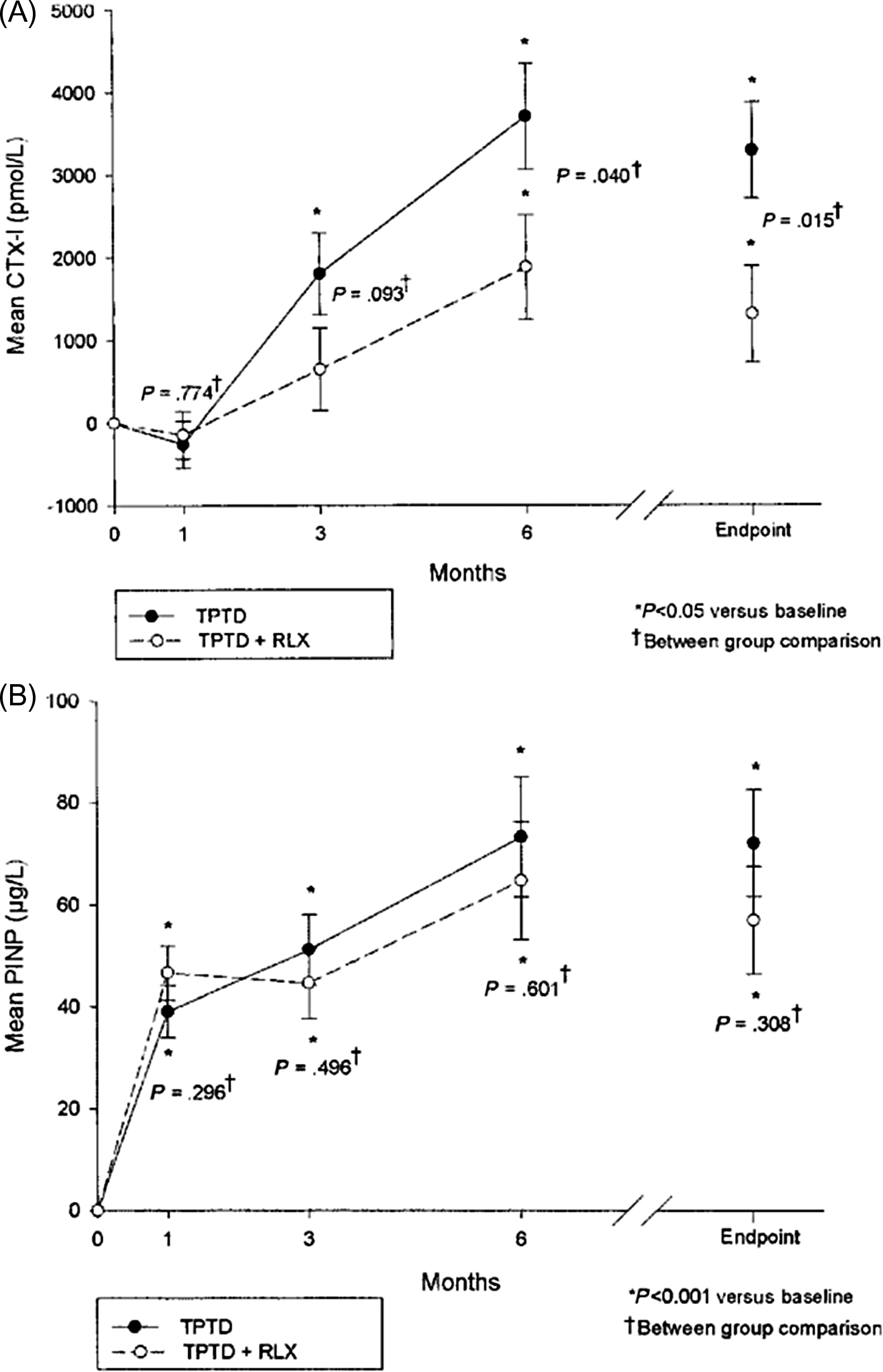
The effect of PTH on BTMs after antiresorptive treatment depends on the degree of bone turnover inhibition by the antiresorptive therapy. After raloxifene therapy the teriparatide-induced increase in BTMs was faster and greater in magnitude than after alendronate therapy . In women treated with bisphosphonates the effect of teriparatide varied according to the drug. In the women treated with risedronate, BTMs were higher and teriparatide induced a greater increase in BTMs than in alendronate-treated women . In alendronate-treated postmenopausal women the teriparatide-induced increase in PINP was delayed versus women treated with risedronate or etidronate . These differences reflect the greater bone turnover suppression by alendronate than other drugs. However, they should be interpreted cautiously, as they may be related not only to the agent itself but also to the lag time between the two regimens.
Alendronate administered after PTH decreased BTM levels to below baseline values and were similar to those obtained with alendronate alone . Alendronate administered after abaloparatide decreased BTM levels to levels below baseline values and similar to those in women treated with alendronate alone ( Fig. 65.13 ). This inhibition of bone turnover may prevent the loss of the bone built under the anabolic treatment and prolongs its secondary mineralization that results in an additional increase in BMD.
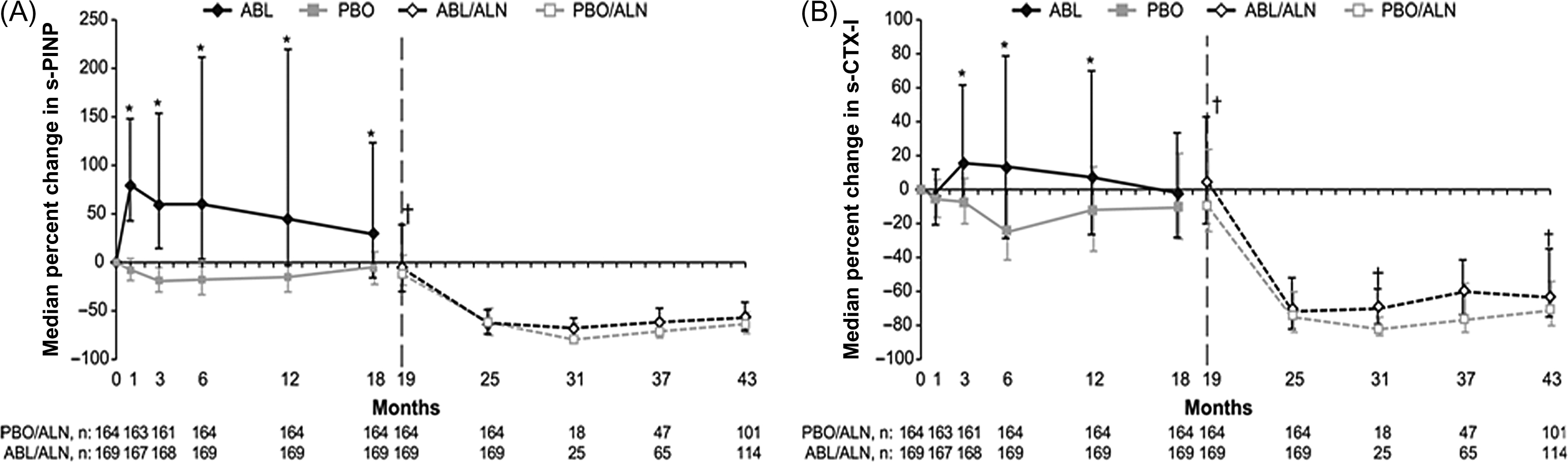
In osteoporotic women on alendronate for ≥1 year, a standard daily PTH-1-34 regimen and alendronate therapy was compared with cyclic PTH-1-34 and alendronate . Cyclic PTH-1-34/alendronate increased bone formation similar to the daily PTH-1-34/alendronate, whereas NTX-I levels were similar to those in the alendronate-treated women. Cyclic PTH-1-34/alendronate is more convenient and provides similar bone gain. After another 1 year on alendronate a retreatment with teriparatide stimulated bone formation similar to the initial teriparatide course .
In postmenopausal osteoporotic women, teriparatide was added after 3 months on denosumab treatment . After the initial decrease associated with denosumab, PINP and serum CTX-I increased but were lower than in patients treated with teriparatide alone. In postmenopausal osteoporotic women treated with teriparatide, denosumab decreased BTM levels (OC, serum CTX-I) to the concentrations similar to those found in women treated with denosumab . In the same study, teriparatide induced a rapid increase in BTM levels in women previously treated with denosumab .
Few studies assessed the administration of an antiresorptive drug after (or with) another antiresorptive drug. In postmenopausal, alendronate-treated women, zoledronate induced an additional decrease in BTM levels after 3 months . BTM levels increased and, 1 year after zoledronate administration, were higher than in women receiving alendronate alone, but in the lower values of the normal range. In postmenopausal women, denosumab administered after alendronate induced an additional decrease in BTMs followed by an increase in BMD .
The effect of denosumab was studied in postmenopausal women who received teriparatide or zoledronate or were treatment-naïve . Denosumab suppressed serum PINP and CTX-I to the same levels regardless of prior treatment and of initial BTM concentrations.
Combination therapy may induce a greater increase in bone strength or stabilize bone gain induced by anabolic treatment using antiresorptive therapy. If possible, it could be more convenient for the patient (e.g., cyclic instead of daily injections). However, the antifracture efficiency of such combination treatment has not been evaluated.
65.5.4.6
Head-to-head comparisons of the antiosteoporotic treatments
Several studies compared two antiosteoporotic regimens. In postmenopausal women HRT plus risedronate therapy decreased BTM levels more than HRT alone . However, the changes in BMD and in bone turnover assessed by histomorphometry were similar in both groups. In postmenopausal women with low BMD ( T -score ≤–2.0), once-weekly alendronate induced a greater decrease in the BTM levels (serum CTX-I, urinary NTX-I, PINP, bone ALP) and a greater increase in BMD compared to once-weekly risedronate . Alendronate and raloxifene were compared in two groups of postmenopausal osteoporotic women . Once-weekly alendronate induced a greater decrease in the BTMs (urinary NTX-I, bone ALP) and a greater increase in BMD versus raloxifene . The combination of alendronate and raloxifene decreased the BTM levels (bone ALP, urinary NTX-I) and increased BMD to a similar extent as alendronate alone, but more than raloxifene alone .
Several studies compared denosumab with bisphosphonates in postmenopausal women. In women with low BMD ( T -score≤–2.0), denosumab induced a greater decrease in the BTMs (PINP, CTX-I) and a greater rise in BMD versus alendronate . In women suboptimally adherent to alendronate, denosumab induced a greater decrease in the BTM levels and a greater increase in BMD versus risedronate . In women who had been treated previously with oral bisphosphonates, denosumab decreased serum CTX-I more than risedronate or ibandronate that was followed by a higher increase in BMD . Denosumab and zoledronate (standard doses) were compared in osteoporotic women treated previously with oral bisphosphonate . Both agents induced a similar short-term decrease in bone resorption and formation. However, in the denosumab-treated women, BTM levels remained low and stable, whereas in the zoledronate-treated group, BTM levels returned to baseline. These different changes in BTM levels were associated with a higher increase in BMD in the denosumab-treated group.
Zolendronate and risedronate were compared in glucocorticoid-treated patients . Zoledronate induced a greater and more rapid decrease in bone resorption followed by a slightly greater decrease in bone formation. During treatment (6–12 months), bone resorption markers were lower in the zoledronate-treated patients. Denosumab and risedronate were compared in patients with glucocorticoid-induced osteoporosis . Denosumab induced a greater decrease in serum PINP and CTX-I concentrations compared to risedronate, which was followed by a greater increase in BMD in this group.
Abaloparatide and teriparatide were compared in two groups of postmenopausal women with osteoporosis . Abaloparatide induced a smaller increase in the BTM levels, but a greater increase in hip BMD versus teriparatide.
Romosozumab and teriparatide were compared in postmenopausal women previously treated with bisphosphonates . Teriparatide induced a greater increase in serum PINP and CTX-I ( Fig. 65.14 ). By contrast, romosozumab induced a greater increase in BMD and a greater reduction in fracture incidence. In postmenopausal osteoporotic women, romosozumab and alendronate induced the expected changes in serum PINP and CTX-I, which were associated with a greater increase in BMD in the romosozumab-treated group . After 1 year, both groups received alendronate that was associated with decreased BTM levels (vs baseline), stabilization of BMD, and lower fracture incidence in the romosozumab-alendronate group.
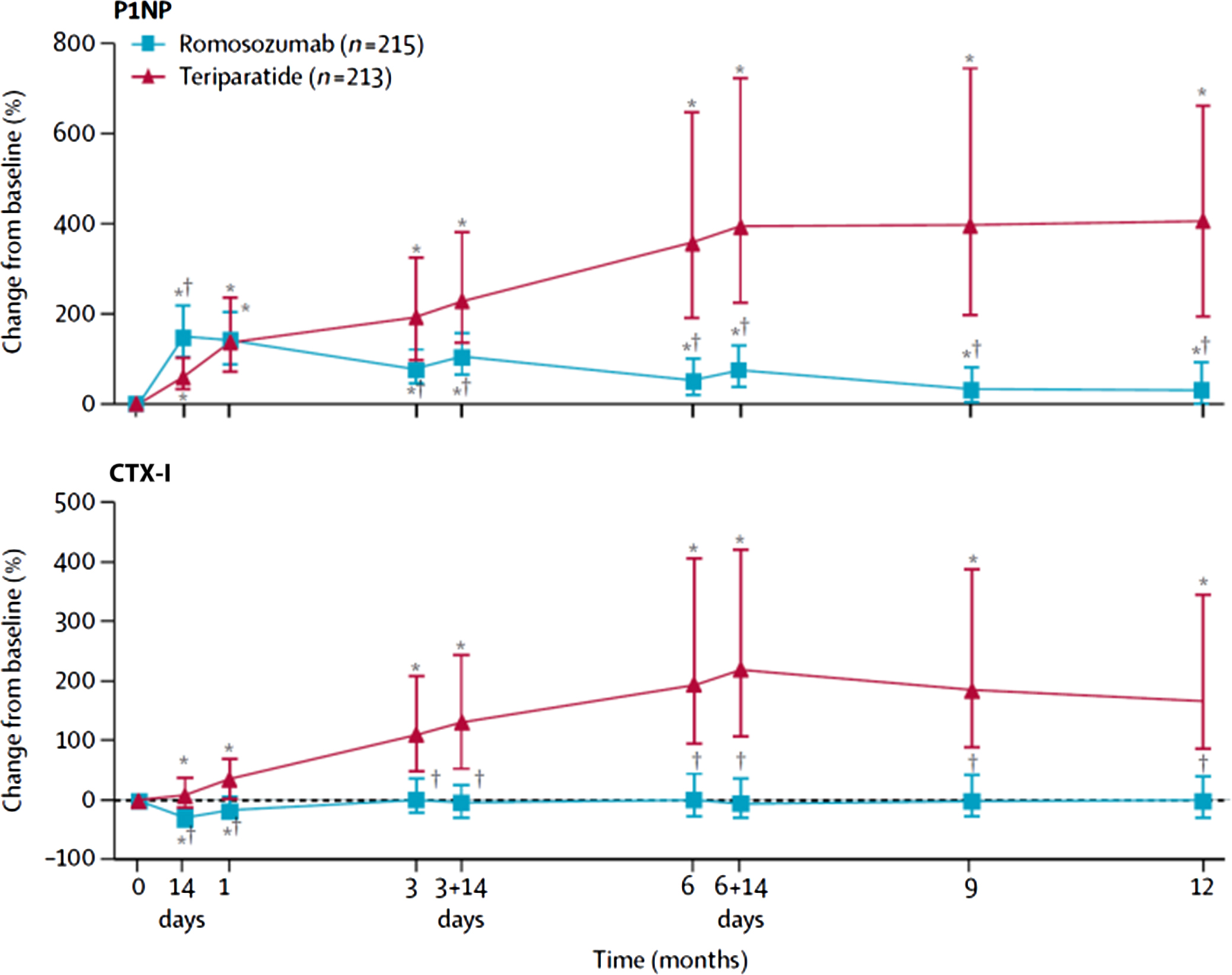
The head-to-head studies provide insight into the effect of antiosteoporotic drugs on bone. Stronger inhibitors of bone resorption also induce a greater increase in BMD. However, the associations between the decrease in the BTM levels and that in fracture risk are not consistent. Zoledronate and denosumab decrease BTM levels and fracture incidence more than risedronate. By contrast, alendronate decreases BTM levels more than risedronate, but both drugs reduce vertebral fracture incidence to a similar extent. Thus the antiresorptive medications may reduce fracture risk by mechanisms that are not completely captured by BTMs or BMD. The increase in bone formation elicited by various anabolic medications is not paralleled by an increase in BMD. It implies that the increase in BMD induced by these drugs may depend rather on the balance between the increased bone formation and bone resorption (elevated or depressed).
65.5.4.7
Potential utility of bone turnover markers for bridging studies
The aim of bridging studies is to show that a new regimen of a drug is not less effective than a reference regimen through the use of surrogate markers. The major advantage is lower cost compared with a trial based on antifracture efficacy without the loss of clinical value of the data. Similar changes in the surrogate markers in two groups treated with two regimens of one drug suggest that they have similar efficacy. In bridging studies, BTMs are an auxiliary tool. They are helpful, as the therapy-induced reduction of the fracture risk and changes in BTM levels are correlated. This approach has been accepted by the European Agency for Evaluation of Medicines and the Group for the Respect of Ethics and Excellence in Science .
Bridging studies permitted approval for once-weekly and once-monthly regimens of bisphosphonates. Weekly versus daily regimens of alendronate and risedronate decreased BTM levels and increased BMD in a similar way . Intermittent risedronate (75 mg on two consecutive days of the month or 150 mg once-monthly) provided similar effects on BMD and BTMs as 5 mg daily . Monthly and intermittent regimens of oral ibandronate provided similar effects on BMD and BTMs as daily dosing . Bazedoxifene was as effective in the Japanese population as it had been in the pivotal trial .
Comparison of BTM levels during oral and intravenous treatment or for different intravenous doses is more complex. I.V. treatment decreases bone resorption rate . The early decrease is similar for various doses, whereas the duration of this decrease and its degree in the later phase is dose-dependent. The decrease in bone formation starts later and is progressive. The temporal pattern of BTM changes, especially the magnitude of the reduction of the rate of bone resorption at the end of the dosing interval, should repeat those observed with the original regimen in order to induce the same effect on BMD and bone strength .
65.5.4.8
Bone turnover markers and treatment monitoring at the individual level
Low compliance is a problem during antiosteoporotic treatment limiting the antifracture efficacy . BTM levels correlated with adherence because of rapid and substantial changes during treatment, and their use to identify nonadherence to oral bisphosphonates has been proposed by IOF and ECTS working groups . Given that greater treatment-related decreases in BTMs are associated with better antifracture efficacy, it has been suggested that during antiresorptive treatment, the BTM level should be within the lower half of the reference interval for healthy premenopausal women .
Better adherence is associated with a greater decrease in BTM levels, but this link is weak and not clinically useful . In older women on antiresorptive therapy a randomized trial of NTX-I monitoring did not improve persistence compared with usual care . In contrast, persistence was better in women receiving positive feedback (greater decrease in the NTX-I level). However, the impact of this feedback is not straightforward—women who had a greater decrease in NTX-I and were more adherent after the feedback could have been more adherent from the beginning. In addition, these studies recruited volunteers who tend to be more compliant than the general population. Thus further studies are needed under conditions more similar to real clinical practice to check whether measurement of BTMs may improve persistence with antiosteoporotic treatment.
65.5.4.9
Potential use of bone turnover markers in the assessment of the risk of complications of antiresorptive therapy
Atypical femoral fracture is associated with antiresorptive therapy in osteoporotic patients. Some studies tried to establish whether BTMs help to identify patients at high risk of this fracture. However, the data were not conclusive. In most cases, biological samples were collected after the fracture and the interval after fracture and degree of fracture healing were variable. The patients had taken various types of bisphosphonate and many patients were still taking them . In five patients from a trial on bisphosphonates, most of the BTMs levels before the atypical femoral fracture occurred were in the normal range .
Osteonecrosis of the jaw (ONJ) is rare in patients receiving doses of bisphosphonates or denosumab for osteoporosis . Studies in small groups showed that patients with ONJ had low BTM levels (CTX-I, bone ALP) . However, patients taking bisphosphonates (osteoporosis, cancer) often have low BTM levels. The serum CTX-I, level that was classified as being associated with a higher risk of ONJ (<0.15 ng/mL), is found in 50% of zoledronate-treated patients . The link between low BTMs and ONJ was not confirmed in other studies or there was a large overlap of the individual values .
Thus these data do not support the idea that low bone turnover is a “high risk zone” for atypical femoral fracture or ONJ . No threshold BTM level warrants “drug holidays” or reinitiation of treatment at the individual level. However, these data do not exclude the possibility that reduced bone turnover may contribute to the pathogenesis of the atypical femoral fracture or ONJ .
65.6
Bone turnover markers in men
In young men, BTM levels are high and then decrease with age. After the age of 60, bone resorption increases . Conversely, bone formation was slightly increased, decreased or remained stable, according to the study.
Before the age of 60 the relation between BTM levels and BMD was nonsignificant . It may reflect bone turnover slowdown during the consolidation (formation of peak BMD after growth arrest) . As periosteal apposition and endosteal bone loss are slow in middle-aged men, BMD may depend more on the peak BMD than on the bone turnover rate. After the age of 60, high BTM levels weakly correlated with lower BMD and faster bone loss . As bone loss is slower in men than women, longer follow-up is necessary to obtain an accurate estimate of bone loss. The assessment of bone loss by DXA is biased by the concomitant periosteal apposition. Bone turnover is influenced by many factors, and a single measurement of BTM may not correspond to the average bone turnover rate over a long period of time.
In large cohorts of older men, classical BTMs did not predict fractures after adjustment for age and BMD ( Fig. 65.15 ). However, some studies showed significant links. Low gammacarboxylated OC level predicted fractures; however, the analysis was not adjusted for BMD . In the Dubbo cohort, only ICTP predicted fracture . However, blood collection was not standardized. An increased α-CTX-I/β-CTX-I ratio predicted clinical vertebral fracture in older men after adjustment for hip BMD .