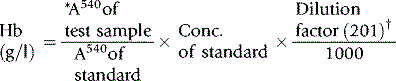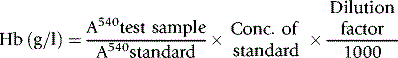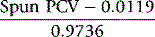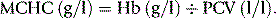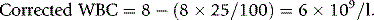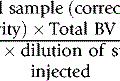Chapter 3 Basic haematological techniques
All the tests discussed in this chapter can be performed on venous or free-flowing capillary blood that has been anticoagulated with ethylenediaminetetra-acetic acid (EDTA) (see p. 6). Thorough mixing of the blood specimen before sampling is essential for accurate test results. Ideally, tests should be performed within 6 h of obtaining the blood specimen because some test results are altered by longer periods of storage. However, results that are sufficiently reliable for clinical purposes can usually be obtained on blood stored for up to 24 h at 4°C (see p. 7).
Haemoglobinometry
The haemoglobin concentration (Hb) of a solution may be estimated by measurement of its colour, by its power of combining with oxygen or carbon monoxide or by its iron content. The methods to be described are all colour or light-intensity matching techniques, which also measure, to a varying extent, any methaemoglobin (Hi) or sulphaemoglobin (SHb) that may be present. The oxygen-combining capacity of blood is 1.34 ml O2 per g haemoglobin. Ideally, for assessing clinical anaemia, a functional estimation of Hb should be carried out by measurement of oxygen capacity, but this is hardly practical in the routine haematology laboratory. It gives results that are at least 2% lower than those given by the other methods, probably because a small proportion of inert pigment is always present. The iron content of haemoglobin can be estimated accurately,1 but again the method is impractical for routine purposes. Estimations based on iron content are generally taken as authentic, but iron bound to inactive pigment is included. Iron content is converted into haemoglobin by assuming the following relationship: 0.347 g iron = 100 g haemoglobin.2
Measurement of haemoglobin concentration using a spectrometer (spectrophotometer) or photoelectric colorimeter
Although the HiCN reagent contains cyanide, there is only 50 mg of potassium cyanide per litre and 600–1000 ml would have to be swallowed to produce serious effects. However, the use of potassium cyanide has been viewed as a potential hazard; alternative nonhazardous reagents that have been proposed are sodium azide3 and sodium lauryl sulphate,4,5 which convert haemoglobin to haemiglobinazide and haemiglobinsulphate, respectively. They are used in some automated systems, but no stable standards are available and they, too, are toxic substances that must be handled with care.
Other methods that have been used include Sahli’s acid-haematin method, which is less accurate because the colour develops slowly, is unstable and begins to fade almost immediately after it reaches its peak. The alkaline-haematin method gives a true estimate of total Hb even if carboxyhaemoglobin (HbCO), Hi or SHb is present; plasma proteins and lipids have little effect on the development of colour, although they cause turbidity. The original method was more cumbersome and less accurate than the HiCN or HbO2 methods, but a modified method has been developed in which blood is diluted in an alkaline solution with non-ionic detergent and read in a spectrometer at an absorbance of 575 nm against a standard solution of chlorohaemin.6,7 One evaluation has given encouraging results,8 although another study has shown a bias of 2.6% when compared with the reference method, with non-linearity in the relationship between haemoglobin concentration and absorbance at high and low haemoglobins.9
Haemiglobincyanide (cyanmethaemoglobin) method
The haemiglobincyanide (cyanmethaemoglobin) method is the internationally recommended method2 for determining the haemoglobin concentration of blood. In some countries cyanide reagents are no longer available. The basis of the method is dilution of blood in a solution containing potassium cyanide and potassium ferricyanide. Haemoglobin, Hi and HbCO, but not SHb, are converted to HiCN. The absorbance of the solution is then measured in a spectrometer at a wavelength of 540 nm or a photoelectric colorimeter with a yellow-green filter (e.g. Ilford 625, Wratten 74, Chance 0 Gr1).
Diluent
The original (Drabkin’s) reagent had a pH of 8.6. The following modified solution listed in Table 3.1, Drabkin-type reagent, as recommended by the International Committee for Standardization in Haematology (ICSH),2 has a pH of 7.0–7.4. It is less likely to cause turbidity from precipitation of plasma proteins and requires a shorter conversion time (3–5 min) than the original Drabkin’s solution, but it has the disadvantage that the detergent causes some frothing.
Table 3.1 Drabkin-type reagent
| Potassium ferricyanide (0.607 mmol/l) | 200 mg |
| Potassium cyanide (0.768 mmol/l) | 50 mg |
| Potassium dihydrogen phosphate (1.029 mmol/l) | 140 mg |
| Non-ionic detergenta | 1 ml |
| Distilled or deionized water | To 1 litre |
a Suitable non-ionic detergents include Nonidet P40 (VWR International, Merck, Eurolab) and Triton X-100 (Aldrich).
Haemiglobincyanide Reference Standard
With the advent of HiCN solution, which is stable for many years, other standards have become outmoded.10 The International Committee for Standardization in Haematology2 has defined specifications on the basis of a relative molecular mass (molecular weight) of human haemoglobin of 64 458 (i.e. 16 114 as the monomer) and a millimolar area absorbance (coefficient extinction) of 11.0 (that is, the absorbance at 540 nm of a solution containing 55.8 mg of haemoglobin iron per litre).
Some standards are prepared from ox blood, which has the same coefficient extinction but a molecular weight of 64 532 (16 133 as the monomer). These specifications have been widely adopted; a World Health Organization (WHO) International Standard has been established and a comparable reference material is available from the ICSH. A new lot of the haemoglobincyanide or haemoglobin standard was released in 2008.11 This newly released standard replaces the previous lot and was produced using the same methodology previously specified by ICSH.2 The current standard has an assigned concentration value of 574.2 (± 5.1) mg/l or 35.63 (± 0.32) μmol/l; the exact concentration is indicated on the label. The stability expectation of this standard is 15 years11 but it will continue to be monitored on a twice-yearly basis over the lifetime of this lot of reference material. The haemoglobin standard provides a reference material from which both laboratory-based cell counters and point-of-care instruments calibrate their haemoglobin methods.2
Calculation of Haemoglobin Concentration
Preparation of Standard Graph and Standard Table
Prepare five dilutions of the HiCN reference standard (or equivalent preparation) (brought to room temperature) with the cyanide-ferricyanide reagent according to Table 3.2. Because the graph will be used to determine the haemoglobin measurements, it is essential that the dilutions are performed accurately.
Table 3.2 Dilutions of haemiglobincyanide (HiCN) reference solution for preparation of standard graph
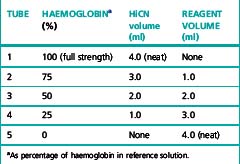
The rate of conversion of blood containing HbCO is markedly slow. This difficulty can be overcome by prolonging the reaction time to 30 min before reading.12 The difference between the 5 and 30 min readings can be used as a semiquantitative method for estimating the percentage of HbCO in the blood.
As referred to earlier, lauryl sulphate5 or sodium azide3 can be used as non-hazardous substitutes for potassium cyanide. However, no stable standards are available for these methods so a sample of blood that has first had a haemoglobin value assigned by the HiCN method needs to be used as a secondary standard.
Abnormal plasma proteins or a high leucocyte count may result in turbidity when the blood is diluted in the Drabkin-type reagent. The turbidity can be avoided by centrifuging the diluted sample or by increasing the concentration of potassium dihydrogen phosphate to 33 mmol/l (4.0 g/l).13
Oxyhaemoglobin method
The HbO2 method is the simplest and quickest method for general use with a photometer. Its disadvantage is that it is not possible to prepare a stable HbO2 standard, so the calibration of these instruments should be checked regularly using HiCN reference solutions or a secondary standard of preserved blood or lysate (see p. 25). The reliability of the method is not affected by a moderate increase in plasma bilirubin, but it is not satisfactory in the presence of HbCO, Hi or SHb.
Standard
A standard should be prepared from a specimen of normal anticoagulated whole blood. Its haemoglobin concentration is first determined by the HiCN method (see p. 25). The blood is then diluted 1:201 by pipetting 20 ml of the well-mixed blood into 4 ml of ammonia; sequential dilutions are made in ammonia and absorbance is read in a spectrometer at 540 nm or photometer using a yellow-green filter (Ilford 625, Wratten 74 or Chance 0 Gr 1). The readings are plotted on arithmetic graph paper. Linearity of response is checked and absorbance is related to haemoglobin from the measurement obtained in the original sample by the HiCN method.
Colorimeters and light filters unfortunately, differ sufficiently to make it essential to check the chosen standard at frequent intervals against a HiCN reference preparation in the photometer in which it is going to be used. It is probably preferable to use a new fresh whole-blood sample each day as a secondary standard after measuring its haemoglobin concentration by the HiCN method. Preserved blood (see p. 599) or lysate (see p. 599) can be used instead.
Direct calculation from a standard:
Direct spectrometry
The haemoglobin concentration of a diluted blood sample can be determined by spectrometry without the need for a standard, provided that the spectrometer has been correctly calibrated. The blood is diluted 1:201 (or 1:251) with cyanide-ferricyanide reagent (see p. 25) and the absorbance is measured at 540 nm. Haemoglobin concentration is calculated as follows:
When assigning a value to a haemoglobin solution that may be used as a reference preparation, it is necessary first to calibrate the spectrometer. This requires checking wavelength with a holmium oxide filter, absorbance with a set of calibrated neutral density filters and stray light with a neutral density filter at 220 nm (National Physical Laboratory, Teddington, UK). Matched optical or quartz glass cuvettes with a transmission difference of <1% at 200 nm should be used. Subsequently, the calibration of the spectrophotometer can be checked by verifying that it gives an accurate reading of the HiCN standard. Slight deviations from the expected A540 HiCN value for the standard may be used to correct the results of test samples for a bias in measurement.2
Direct reading portable haemoglobinometers
Colour Comparators
These are simple clinical devices that compare the colour of blood against a range of colours representing haemoglobin concentrations. They are intended for anaemia screening in the absence of laboratory facilities and are described in Chapter 26.
Portable Haemoglobinometers
The HemoCue system (HemoCue AB, Ängelsholm, Sweden) is a well-established method for haemoglobinometry. It consists of a precalibrated, portable, battery-operated spectrometer; no dilution is necessary because blood is run by capillary action directly into a cuvette containing sodium nitrite and sodium azide, which convert the haemoglobin to azidemethaemoglobin. The absorbance is measured at wavelengths of 565 and 880 nm. Measurements are not affected by high levels of bilirubin, lipids or white cells and it is sufficiently reliable for use as a laboratory instrument; it is easy for non-technical personnel to operate and is thus also suitable for use at point-of-care. The cuvettes must be stored in a container with a drying agent and kept within the temperature range of 15–30°C. Some devices are now available that use reagent-free cuvettes that will not deteriorate in adverse climatic conditions.14 HemoCue have recently released a portable system that measures both haemoglobin and the white blood cell count (WBC), the HemoCue WBC.15
The DiaSpect Haemoglobinometry system is a newly developed technology for measuring haemoglobin concentration in unaltered whole blood in a special plastic cuvette that also serves as the sampling device.16 The instrument is a portable spectrophotometer powered by 3.6 V integrated lithium-ion rechargeable batteries or 100–240 V adaptor. As the cuvettes do not contain any reagents, they are not affected by temperature or humidity and no special storage conditions are required. They have a shelf life of at least 2 years. Haemoglobin fractions are measured from absorbance wavelengths between 400 and 800 nm. A patented method eliminates the impact of scattering from the blood cells while possible background turbidity from interfering substances is measured and compensated for at high wavelength. The results are displayed in <5 seconds. Preliminary studies have shown an accuracy within ± 3 g/l for measurements between 10 and 200 g/l.
Non-invasive Screening Tests
Methods are being developed for using near infrared spectroscopy at body sites, mainly a finger, to identify the spectral pattern of haemoglobin in an underlying blood vessel and derive a measurement of haemoglobin concentration. Early studies have shown an approximate correlation with blood haemoglobinometry.17,18
Range of Haemoglobin Concentration in Health
See Chapter 2, Tables 2.1, 2.2 and 2.3. It should be noted that there are sex differences, diurnal variations and environmental and physiological factors that must also be taken into account.
Packed cell volume or haematocrit
Microhaematocrit Method
The microhaematocrit method19 is carried out on blood contained in capillary tubes 75 mm in length and having an internal diameter of about 1 mm. The tubes may be plain for use with anticoagulated blood samples or coated inside with 1 iu of heparin for the direct collection of capillary blood. The centrifuge used for the capillary tubes provides a centrifugal force of c12 000 g and 5 min centrifugation results in a constant PCV. When the PCV is >0.5, it may be necessary to centrifuge for a further 5 min.
Accuracy of Microhaematocrit
The microhaematocrit method has an adequate level of accuracy and precision for clinical utility.20 However, attention must be paid to a number of factors that may produce an inaccurate result.
Blood Sample
Failure to mix the blood sample adequately will produce an inaccurate result. The degree of oxygenation of the blood also affects the result because the PCV of venous blood is ≈2% higher than that of fully aerated blood (which has lost CO2 and taken up O2).21 To ensure adequate oxygenation and sample mixing, the free air space above the sample should be >20% of the container volume.
Capillary Tubes
Variation of the bore of the tubes may cause serious errors if they are not within the narrow limits of defined specifications that should be met by manufacturers: length 75 ± 0.5 mm; internal diameter 1.07–1.25 mm; wall thickness 0.18–0.23 mm; and bore taper not exceeding 2% of the internal diameter over the entire length of the tube.20
Plasma Trapping
The amount of plasma trapped between red cells, especially in the lower end of the red cell column, and red cell dehydration during centrifugation generally counterbalance each other and the error caused by trapped plasma is usually not more than 0.01 PCV units. Thus, in routine practice, it is unnecessary to correct for trapped plasma, but if the PCV is required for calibrating a blood cell analyser or for calculating blood volume, the observed PCV should be reduced by a 2% correction factor after it has been centrifuged for 5 min or for 10 min with polycythaemic blood.22 It is, however, preferable to use the surrogate reference method.23 Plasma trapping is increased in macrocytic anaemias,24 spherocytosis, thalassaemia, hypochromic anaemias and sickle cell anaemia;25 it may be as high as 20% in sickle cell anaemia if all the cells are sickled.24
International Council for Standardization in Haematology Reference Method
Haemoglobin concentration is measured by the routine method on blood specimens with a range of haemoglobin concentrations. Samples of the same specimens are then taken into special borosilicate glass capillary tubes, which are centrifuged for 5 min or longer to achieve full red cell packing. The tubes are then broken at the midpoint of the packed red cells, blood is extracted with a micropipette and its haemoglobin concentration is measured. PCV is calculated as the ratio of the Hb of whole blood to that of the packed cells. This method26 is appropriate for instrument and reagent manufacturers, but it is time-consuming and requires significant expertise, which makes it impractical for occasional use in routine laboratories. Accordingly, the International Council for Standardization in Haematology (ICSH) has developed a ‘surrogate reference method’.23
Surrogate Reference Method
Equipment
Method
This formula applies only to the specified capillary tubes; other tubes require specific validation by the ICSH reference method22 so that an appropriate formula can be derived. If the surrogate reference measurements are to be used to validate equipment or methods, a minimum of six different blood samples are required, at least two in each of the ranges of PCV 0.20–0.25, 0.40–0.45 and 0.60–0.65. If necessary, the PCV of normal samples may be adjusted by the appropriate addition or removal of autologous plasma.
Manual cell counts and red cell indices
The principles of manual cell counts, the use of the haemocytometer counting chamber for manually counting white cells and platelets and the limitations of these measurements are described in Chapter 26.
An accurate RBC enables the MCV and MCH to be calculated. In well-equipped laboratories, where these indices are provided by an automated system (see p. 41), they are of considerable clinical importance and are widely used in the classification of anaemia. Where automated analysers are not used, manual RBCs (and consequently, calculations of these red cell indices) are so inaccurate and time-consuming that they have become obsolete.
Manual differential leucocyte count
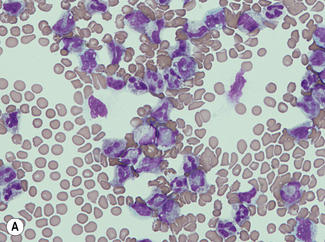
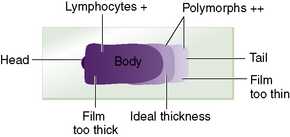
For a reliable differential count on films spread on slides, the film must not be too thin and the tail of the film should be smooth. To achieve this, the film should be made with a rapid movement using a smooth glass spreader. This should result in a film in which there is some overlap of the red cells, diminishing to separation near the tail, and in which the white cells in the body of the film are not too badly shrunken. If the film is too thin or if a rough-edged spreader is used, many of the white cells, perhaps even 50% of them, accumulate at the edges and in the tail (Fig. 3.1). Moreover, a gross qualitative irregularity in distribution is the rule: polymorphonuclear neutrophils and monocytes predominate at the margins and the tail; lymphocytes predominate in the middle of the film (Fig. 3.2). This separation probably depends on differences in stickiness, size and specific gravity of the different types of cells.
Method
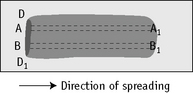
Count the cells using a ×40 objective in a strip running the whole length of the film. Avoid the lateral edges of the film. Inspect the film from the head to the tail and if fewer than 100 cells are encountered in a single narrow strip, examine one or more additional strips until at least 100 cells have been counted. Each longitudinal strip represents the blood drawn out from a small part of the original drop of blood when it has spread out between the slide and spreader (Fig. 3.3). If all the cells are counted in such a strip, the differential totals will closely approximate the true differential count. This technique is liable to error if cells in the thick part of the film cannot be identified; also, it does not allow for any excess of neutrophils and monocytes at the edges of the film, but this preponderance is slight in a well-made film and in practice makes little difference to the result.
Basophil and eosinophil counts
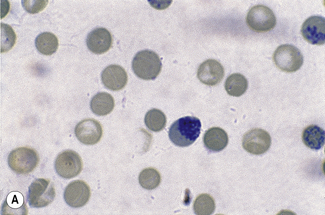
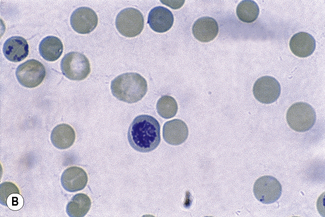
A manual basophil or eosinophil count may be necessary to validate an automated count or when abnormal characteristics of the cells render an automated count unreliable, e.g. with degranulated eosinophils. Count the percentage of eosinophils or basophils in a differential count of all the leucocytes on a stained blood film. If the cells of interest are infrequent, a 500-cell differential count should be performed. If fewer than 500 cells are seen in the film, continue the count on a second film. However, if the eosinophil count is markedly elevated a conventional 100-cell count will suffice for most purposes. Calculate the eosinophil or basophil count per litre from the total leucocyte count. It is essential to have thin, preferably short, films with the leucocytes evenly distributed throughout the film and readily identified (see p. 31).
Range of Eosinophil Count in Health
See Chapter 2, Tables 2.1, 2.2 and 2.3.
There is normally considerable diurnal variation in the eosinophil count and differences amounting to as much as 100% have been recorded. The lowest counts are found in the morning (10 a.m. to noon) and the highest at night (midnight to 4 a.m.).27,28 For a review of the causes of eosinophilia, see Brito-Babapulle.29
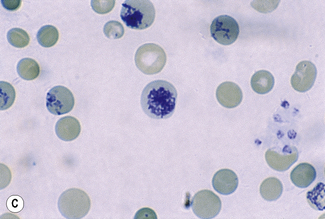
Reporting the Differential Leucocyte Count
The differential count, expressed as the percentage of each type of cell, should be related to the total leucocyte count and the results should be reported in absolute numbers (× 109/l). Myelocytes and metamyelocytes, if present, are recorded separately from neutrophils. Band (stab) cells are generally counted as neutrophils, but it may be useful to record them separately. They normally constitute <6% of the neutrophils; an increase may point to an inflammatory process even in the absence of an absolute leucocytosis.32 However, the band cell count is imprecise and, although it is sometimes recommended in infants, it has been found to be unhelpful in predicting occult bacteraemia in this group.33
Reference Differential White Cell Count
A reference method is required to validate the accuracy of automated systems34 (described later). The method that has been used widely for this purpose is essentially similar to the routine manual procedure on stained blood films, but to ensure adequate precision a 200-cell count is carried out by two independent observers, each on two films prepared from the same sample. However, this is still too imprecise for cells with a low frequency; attempts have been made to establish a reference method using flow cytometry with specific monoclonal-antibody labelling of the specific cell types including immature leucocytes.35,36 More recent flow cytometric protocols also include blast cells, reactive lymphocytes, differentiation between B and T lymphocytes and nucleated red cells.37,38
Platelet count
The method for manual counting of platelets using a counting chamber is described on p. 610. If an RBC by a semiautomated counter is available, it is possible to obtain an approximation of the platelet count by counting the proportion of platelets to red cells in a thin part of a film made from an EDTA-anticoagulated blood sample, using the ×100 oil-immersion objective and, if possible, eyepieces provided with an adjustable diaphragm, as for a reticulocyte count.
Reticulocyte count
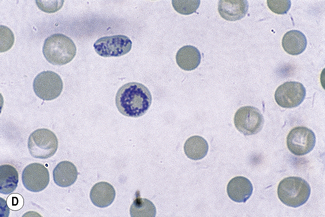
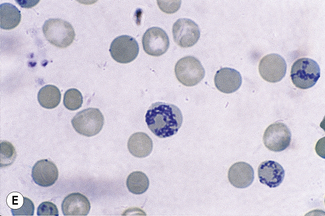
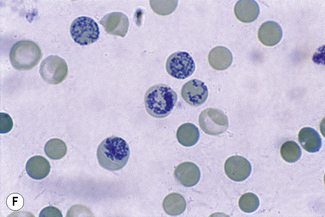
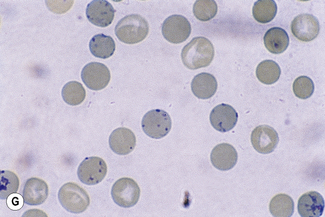
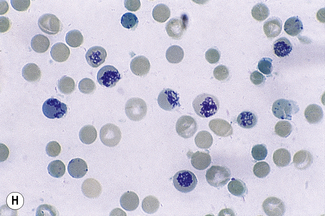
This reaction takes place only in vitally stained unfixed preparations. Stages of maturation can be identified by their morphological features. The most immature reticulocytes are those with the largest amount of precipitable material; in the least immature, only a few dots or short strands are seen. Reticulocytes can be classified into four groups, ranging from the most immature reticulocytes, with a large clump of reticulin (group I), to the most mature, with a few granules of reticulin (group IV) (Fig. 3.4).
Complete loss of basophilic material probably occurs in the bloodstream and, particularly, in the spleen after the cells have left the bone marrow.39 This maturation is thought to take 2–3 days, of which about 24 h are spent in the circulation.
The number of reticulocytes in the peripheral blood is a fairly accurate reflection of erythropoietic activity, assuming that the reticulocytes are released normally from the bone marrow and that they remain in circulation for the normal time period. These assumptions are not always valid because an increased erythropoietic stimulus leads to premature release into the circulation. The average maturation time of these so-called ‘stress’ or stimulated reticulocytes may be as long as 3 days. In such cases, a higher than normal proportion of immature reticulocytes will be found in circulation. A more precise assessment of reticulocyte maturation is possible by quantitative flow cytometry of their RNA content. Nevertheless, adequate information is usually obtained from a simple reticulocyte count recorded either as a percentage of the red cells or, preferably, when the RBC is known, as an absolute number per litre. When there is severe anaemia, the reticulocyte count should be corrected for the anaemia and expressed as a reticulocyte index.40