Jeffrey A. Gelfand, Edouard G. Vannier
Babesia Species
Babesiosis is caused by protozoa parasites of the genus Babesia. Wild and domestic animals are natural reservoir hosts for Babesia, and transmission of Babesia to humans is accidental. Typically a mild flulike illness in young and healthy individuals, babesiosis may be life threatening in the elderly and in asplenic or immunocompromised patients.
The first recorded mention of babesiosis is believed to date to biblical times. In the Book of Exodus, the fifth plague is described as “very grievous murrain” that fell “upon thy cattle which is in the field, upon the horses, upon the asses, upon the camels, upon the oxen and upon the sheep.” Still nowadays, bovine babesiosis is referred to as murrain in the Irish countryside. In 1888, the microbiologist and pathologist Victor Babes attributed to an intraerythrocytic microorganism the febrile hemoglobinuria and death of cattle in Romania.1,2 Shortly thereafter, Theobald Smith and Frederick Kilborne described a similar piroplasm (L. pirum, “pear”) in the erythrocytes of Texas cattle with fever. Initially named Pyrosoma, this organism became known as Babesia bigemina. They also identified the tick Rhipicephalus annulatus as its vector of transmission, establishing for the first time that hematophagous arthropods can transmit an infectious agent to a vertebrate host. The first well-documented case of human babesiosis was reported in 1957. A 33-year-old splenectomized herdsman had been grazing cattle on tick-infested pastures near Zagreb, Croatia. The infection was fulminant and fatal. Originally identified as Babesia bovis, the causative agent was later reported to be Babesia divergens, a parasite of cattle. In 1969, a 59-year-old woman who had summered on Nantucket Island off the coast of Massachusetts presented with a history of fever, headache, and crampy abdominal pain.3 This patient had an intact spleen, and the causative agent was Babesia microti, a parasite that infects white-footed mice. Andrew Spielman and colleagues identified the vector as the deer tick Ixodes dammini (now referred to as Ixodes scapularis).4 As the number of cases on the island grew rapidly, the disease became known as “Nantucket fever.” Babesiosis caused by B. microti is now recognized as endemic on the mainland of the United States, particularly along the northeastern seaboard and in the upper Midwest. Outside the United States, human babesiosis is sporadic but distributed worldwide, particularly in regions with a temperate climate.2
Epidemiology
United States: Babesia microti
Geographic Distribution
Islands off the southern coast of New England are highly endemic for babesiosis caused by B. microti, and include Nantucket, Martha’s Vineyard, and the Elizabeth Islands (Massachusetts), Block Island (Rhode Island), and Shelter Island, eastern Long Island, and Fire Island (New York). On the mainland, babesiosis is highly endemic from Cape Cod, Massachusetts, to the coastal counties of western Rhode Island and eastern Connecticut, in the counties east of the Hudson River in the Lower Hudson Valley (New York), and in the south-central counties of New Jersey. Areas of low endemicity include the southeastern coastal counties of Maine and New Hampshire and counties in Pennsylvania, Delaware, and Maryland. As with Lyme disease, babesiosis caused by B. microti is highly endemic in the upper Midwest, particularly in Minnesota and Wisconsin. A few cases have been reported in Indiana. Since January 2011, cases of human babesiosis that are identified in the seven states with well-established foci of zoonotic transmission (Connecticut, Massachusetts, Minnesota, New Jersey, New York, Rhode Island, Wisconsin) and in 12 other jurisdictions (California, the District of Columbia, Delaware, Indiana, Maine, Maryland, Nebraska, New Hampshire, Oregon, Tennessee, Vermont, Washington) are to be reported to the Centers for Disease Control and Prevention (CDC).5 Notification is made via the National Notifiable Diseases Surveillance System (NNDSS) using the standard case definition developed by the CDC and the Council of State and Territorial Epidemiologists.
Incidence and Prevalence
The incidence of babesiosis has steadily increased over the past 25 years. In New York State, the first state to mandate the reporting of babesiosis, more than 2700 cases have been notified to the health authorities. About 640 cases were reported from 1986 to 2001, and more than 2100 cases were reported in the following decade. The sharp increase noted since 2005 results from the emergence of babesiosis in the Lower Hudson Valley (Dutchess, Putnam, and Westchester counties) combined with the maintenance of highly endemic foci on eastern Long Island (Nassau and Suffolk counties).6 Along the northeastern seaboard, babesiosis has emerged in areas that were once at the periphery of the “historical heartland,” namely Maine and New Hampshire to the north and Delaware and Maryland to the south. In the upper Midwest, particularly in Minnesota, the number of annual cases has been higher since 2005. Babesiosis caused by B. microti is now considered an emerging infectious disease in the United States.2
The emergence of babesiosis has been attributed to a cascade of environmental changes that have favored deer and ticks.2 Owing to the lack of natural predators and the public outcry over hunting, deer herds have become larger. Because ticks feed on deer, tick populations have thrived. As winters have become milder, ticks have survived in greater numbers. Culling deer herds was proposed, but this measure has been difficult to implement. Tick repellents are available, but their use has been insufficient to prevent the rise of human babesiosis.
The seroprevalence of antibodies against B. microti antigen varies by year, study site, and sample population but has consistently been high in endemic areas, such as Nantucket (1.9% among residents and visitors),7 Block Island (9% among residents),8 southern Connecticut (0.5% to 9% among blood donors),8,9 Shelter Island (4.3% in blood donors; 4.4% to 6.9% among residents),10 and eastern Long Island (1% among residents).11 In Minnesota, seroprevalence among blood donors has been reported at 2%.12 Given that seroprevalence is much higher than prevalence of clinical babesiosis, asymptomatic infection is more common than has been recognized.7 At some sites, however, symptomatic infection may be the rule. A 10-year serosurvey and case finding study of Block Island residents revealed that 20% of adults and 40% of children infected with B. microti are asymptomatic or experience an illness that is so mild that medical attention is deemed unnecessary.8
Modes of Transmission
Tick Bite
B. microti is primarily acquired during the blood meal of the tick I. scapularis,13 but two thirds of people who experience Babesia infection do not recall a tick bite.14,15 The nymphal stage is the primary vector for transmission and is most active from late spring to early summer (see “Microbiology”).13,16 Because the latency period typically lasts from 1 to 6 weeks, half of cases are diagnosed in July and a fourth in August.14,17 Some cases present as early as late spring. Cases that present in late summer or early fall may have acquired the infection from a nymph or an adult tick.
Transfusion
B. microti can unwittingly be transmitted via transfusion of blood products from asymptomatic donors.18 The median interval from transfusion to onset of symptoms is 37 days (range, 11 to 176 days).19 Consistent with the seasonality of tick-borne babesiosis, three fourths of transfusion-transmitted babesiosis (TTB) cases are diagnosed from June through November, with a fifth in August alone. Because asymptomatic infection can persist for months, TTB may be diagnosed throughout the year. Most TTB cases (87%) are diagnosed in residents of highly endemic states (see earlier list). Outside the endemic areas, TTB typically involves contaminated blood products that were imported from an endemic area or were derived from a blood donation by an individual who had returned from an endemic area.
Nearly 170 cases have been identified since the first case was reported in 1979, and three fourths of these cases have occurred since 2000.19–21 In line with the host cell specificity of Babesia species, most cases involve packed red blood cells (RBCs); only four cases have been attributed to platelet units contaminated with residual RBCs.19 By computing cases reported to its national hemovigilance program from 2005 to 2007, the American Red Cross estimated the nationwide risk for TTB at 1.1 case per million red blood cell (RBC) units.22 The risk for TTB has varied by year and area of blood donation. A retrospective study conducted in Rhode Island established that the risk for TTB was approximately 1 case per 15,000 RBC units transfused from 1999 through 2007.23 Consistent with the recent emergence of tick-borne babesiosis, this risk was approximately 1 case per 35,000 units from 1999 to 2001 but 1 case in 12,000 units from 2004 to 2006 and 1 case in 5000 units in 2007. In the neighboring state of Connecticut, the risk for acquiring TTB from a RBC unit was estimated at 1 case per 60,000 units distributed from 2005 through 2007.22 Earlier estimates had ranged from 1 in 601 to 1 in 100,000.18 Transfusion risks are difficult to assess because TTB is underreported or missed. In “look-back” investigations carried out in Connecticut between 1999 and 2000 when B. microti–seropositive donations were withdrawn only if B. microti DNA was detected by PCR, the chance of acquiring B. microti from a seropositive donation was estimated at 33%.24 Given that approximately 150,000 units of blood are donated each year in Connecticut and that the seroprevalence for B. microti is approximately 1.2% in this region, the annual number of potential TTB cases was estimated at about 600. Since the deferral of seropositive index donations was instituted in late 2000, “look-back investigations” in these highly endemic areas have revealed a sharp decrease in the rate of B. microti transmission via the blood supply.24
Transplacental/Perinatal Transmission
The first case of perinatal babesiosis was reported in 1987. Four subsequent cases raised the possibility of transplacental transmission.15 In all five cases, infants were born at term and had not been exposed to ticks. Symptoms developed between the third and the sixth week of life. At time of diagnosis, parasitemia ranged from 2% to 15%. All infants, like their mothers, were seropositive for B. microti. For one infant, B. microti antibodies were detected in a heelstick blood sample obtained on the third day of life.15 In addition, B. microti DNA was amplified from the mother’s placenta. The definitive proof of vertical transmission came from a recent case.25 The mother had been hospitalized for fever and rigors during the 30th week of gestation. Symptoms and laboratory parameters were consistent with babesiosis. On Giemsa-stained blood smear, parasitemia was assessed at 8.6%. Rare intraerythrocytic parasites were also noted in the amniotic fluid. On the second day of hospitalization, the mother underwent cesarean section owing to fetal distress. Immediately after delivery, blood was obtained from the neonate. Intraerythrocytic parasites were observed on a blood smear, and low titers (1 : 32) of B. microti IgG were detected in serum. In addition to vertical transmission, babesiosis has been diagnosed in neonates after tick bite or blood transfusion.26
Risk Factors
Babesiosis caused by B. microti often is severe in individuals who present the following risk factors: age older than 50 years,14,17,27 splenectomy,14,17,28,29 HIV infection,28,30,31 malignancy,28 blockade of tumor necrosis factor-α (TNF-α) activity (infliximab, etanercept),32,33 and immunosuppression associated with therapies for transplantation or cancer (particularly those using rituximab because it depletes B cells).28,34 These risk factors pertain to the transmission of B. microti by ticks as well as blood transfusion. In the latter setting, given that most cases have a significant comorbidity, risk factors for TTB include conditions that require blood transfusion such as hematologic disorders, cardiovascular surgery or procedure, gastrointestinal disease, bleeding and surgery, and complications of prematurity.19,22 Two thirds of TTB patients are older than age 50 years, but two thirds of those younger than age 40 years have a hereditary hematologic disorder, such as sickle cell disease, thalassemia major, or Diamond-Blackfan anemia.19
United States: Other Babesia Species
B. duncani and B. duncani–type organisms are the etiologic agents of human babesiosis along the northern Pacific Coast.35 The index case (WA1) occurred in a 41-year-old normosplenic man from a forested area of south-central Washington State.36 Four cases (CA1 to CA4), all splenectomized men in their 20s to 40s, were subsequently reported in the San Francisco Bay area.37 The first case of TTB caused by B. duncani involved a 76-year-old normosplenic man who had received RBCs donated by a 34-year-old Washington State resident.38 The second case was diagnosed in a premature male infant who received RBCs for anemia of prematurity.39 The donor lived in the San Francisco Bay area and had vacationed in a rural area of central Oregon 3 months before donating. The third case involved a 59-year-old patient who had sickle cell disease and required RBC exchange transfusion.40 The 67-year-old donor reported a possible tick bite during a hike in the San Francisco Bay area. To date, the vector for transmission of B. duncani remains unknown. Seroprevalence against B. duncani antigen ranges from 3.9% (among the enlisted men at the military fort where the CA1 case occurred; titers >1 : 320) and 4.8% (among neighbors of the WA1 case; titers >1 : 256) to 16.9% (south of the site where CA3 was acquired; titers >1 : 320). The incidence of B. duncani infection remains unclear because clinical cases have been too few to validate the serologic test.
Cases caused by B. divergens–like organisms have been documented in Missouri, Kentucky, and Washington State.41–43 All three patients had been splenectomized and were older than age 50 years. These men engaged in outdoor activities but did not recall a tick bite. In two cases, the organisms were identical to piroplasms found in eastern cottontail rabbits, implying that Ixodes dentatus may be a vector for zoonotic transmission of B. divergens–like organisms.44 In Tennessee, one case may have been caused by B. odocoilei, a parasite of white-tailed deer.45
Europe: Babesia divergens
About 30 cases have been attributed to B. divergens, a parasite of cattle that is transmitted by the tick Ixodes ricinus.46–48 Most cases occurred between May and October in France, Ireland, and Great Britain, particularly from regions with cattle. Isolated cases have been reported in Portugal, Spain, Sweden, Finland, and Croatia (including the index case).2 Patients are typically residents of rural areas (farmers, foresters) or vacationers (campers, hikers, hunters). Nearly all patients are splenectomized and experience a severe illness; one had a rudimentary spleen. One mild case occurred in a normosplenic patient.49 Transfusion-transmitted babesiosis caused by B. divergens has not been reported, but antibodies against B. divergens have been detected in healthy blood donors (0.8% in midwestern Germany) and in individuals with clinical or serologic Lyme borreliosis (4% in midwestern Germany; 13% in southeastern Sweden).50,51
Europe: Other Babesia Species
Cases of B. venatorum infection have been reported in Italy, Germany, and Austria.34,52,53 All four patients were men older than 50 years of age. Three had undergone splenectomy for Hodgkin’s disease and one for hairy cell leukemia. One patient had just begun chemotherapy for stage IIIA diffuse large B-cell lymphoma, whereas another was receiving rituximab for reactivation of Hodgkin’s disease. In three cases, a tick bite was the suspected mode of transmission. To date, the prevalence of asymptomatic infection remains unknown because B. venatorum antigen is not available and because B. venatorum sera recognize B. divergens antigen.52 B. venatorum is most closely related to B. odocoilei, with which it forms a sister group to B. divergens.52 Attributed to B. divergens based on morphology and seroreactivity, some of the early cases of babesiosis may have been caused by B. venatorum. A recent asymptomatic case was identified in Poland, but the etiologic agent could not be conclusively identified as B. venatorum (or B. divergens) based on partial sequencing of the 18S rRNA gene.54
The only case of B. microti infection autochthonous to Europe was reported in 2007 from Germany.55 A 42-year-old woman presented with fever and chest pain 1 week after a first cycle of chemotherapy for acute myeloid leukemia (FAB M3). Intraerythrocytic parasites were observed on Giemsa-stained blood smears and were unequivocally identified as B. microti by full sequencing of the 18S rRNA gene. The patient had received numerous platelet concentrates during the 2-month period before diagnosis of babesiosis. The platelet concentrate transfused 6 days before diagnosis had been prepared from a blood donation by an individual who subsequently tested seropositive for B. microti. In support of the transmission of B. microti by contaminated blood products in Europe, serologic evidence of asymptomatic infection has emerged from midwestern Germany (1.7% among healthy blood donors; 8.3% among Lyme borreliosis patients and asymptomatic individuals with a positive Lyme borreliosis serology) and eastern Switzerland (1.5% among community residents).50,56 Symptomatic B. microti infections have been documented in Austria, Czechoslovakia, France, and the Netherlands, but the patients most likely became infected while traveling in the northeastern United States.
Other Regions
A case of B. divergens–like infection was diagnosed in a splenectomized resident of the Canary Isles.2,48 On the African continent, isolated cases have been reported in Egypt, Mozambique, and South Africa.2 A case of U.S.-type B. microti has been documented in Australia.57 In Japan, a patient became ill after a transfusion of blood donated by an asymptomatic resident of the nearby Awaji Island. The organism (Kobe-type) was closely related to U.S.-type B. microti.58 B. microti–like organisms were also implicated in two cases in Taiwan.59,60 In South Korea, a 75-year-old splenectomized woman who presented with fever and severe anemia was infected with a large Babesia species (KO1) that is closely related to organisms found in sheep.61 Three asymptomatic individuals were identified in the patient’s village. Cases caused by B. microti have been reported from western China, along the border with Myanmar.61a Asymptomatic infections with Babesia spp. that infect domestic animals have been reported from Mexico (B. bigemina, B. canis) and Colombia (B. bigemina, B. bovis).2
Microbiology
Babesia spp. are protozoa parasites classified in the phylum Apicomplexa, class Aconoidasida, order Piroplasmidora, and family Babesiidae.47,48 In addition to Babesiidae, the order Piroplasmidora includes Theileriidae. As for Theileria, the intraerythrocytic form of Babesia spp. can present a pear-shape appearance, hence the name of piroplasm. More than 100 Babesia spp. have been reported to infect vertebrates, including mammals and birds.35,47,48,52 Babesia organisms are often acquired during the blood meal of an infected hard-bodied tick of the genus Ixodes. To date, the life cycles of B. microti and I. scapularis are best understood.
Life Cycle of Babesia microti
Acquisition by the Tick
The life cycle of I. scapularis has three active stages (larva, nymph, adult) and requires 2 years for completion.2,13,47,48 In the fall, adult ticks feed primarily on white-tailed deer (Odocoileus virginianus). Deer are incompetent reservoir hosts for B. microti but are essential for maintenance of I. scapularis. Adult ticks overwinter in an engorged state and lay eggs in the spring. Because B. microti does not reach the ovaries of adult ticks should they be infected, eggs are free of B. microti (no transovarial transmission). Larvae hatch in late July and become infected as they feed in the fall on a wide range of vertebrates. The primary reservoir host for B. microti is the white-footed mouse (Peromyscus leucopus).13 Other competent reservoirs include short-tailed shrews, eastern chipmunks, and raccoons.62 Passerines, raptorial birds, and ground-dwelling birds (e.g., wood thrush, veery) are also reservoirs, although of lesser competence. Fed to repletion, larvae overwinter and molt into nymphs in late spring. If the larva is infected, so will be the nymph (trans-stadial transmission). Nymphs feed on P. leucopus and other vertebrates from May through July. The feeding of nymphs in early summer ensures that P. leucopus mice become reservoirs of B. microti for larvae that feed in late summer. In the fall, nymphs molt into adults.
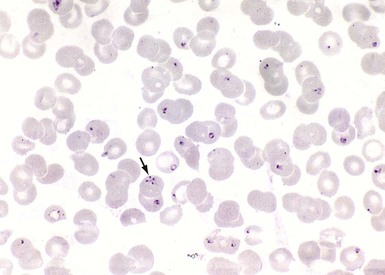
Soon after attachment of the larva, B. microti–infected erythrocytes accumulate in its gut.47,48,63 Pregametocytes mature into gametocytes. A novel endocytic organelle, the cytostome, forms. Microtubules accumulate at the anterior end of the organism to form a raylike structure that contributes to the fusion of gametocytes into zygotes. Using an arrowhead structure, zygotes penetrate the gut epithelium. The arrowhead structure dissociates, and zygotes translocate to the basal lamina. In the hemolymph, zygotes become ookinetes that travel to the salivary acini. There, ookinetes undergo hypertrophy into sporoblasts that remain dormant as long as the larva overwinters.
Transmission to Vertebrate Hosts
Larvae, nymphs, and adult ticks all feed on humans, but the nymph is the primary vector for B. microti transmission.13,47,48 The small size of the nymph (<2.5 mm), its pale gray ground color, the inconspicuous feeding site, and the benign local reaction explain why recollection of a tick bite is uncommon.14,15 As nymphs attach to the host, their temperatures rise. Sporogony is initiated, and the sporoblast membrane folds. After 48 hours, micronemes and rhoptries appear. Cytoplasm starts to bud from the parent sporoblast, and nuclear division ensues. During the last hours of feeding, which typically lasts 72 hours, as many as 100,000 sporozoites are deposited in the dermis of the host. Events that lead sporozoites toward the bloodstream are not well understood. Babesia sporozoites do not undergo merogony in an exoerythrocytic compartment but readily invade erythrocytes. Surface antigens mediate their initial attachment to the erythrocyte.64 The erythrocyte membrane invaginates, leading to the formation of a parasitophorous vacuole that gradually disintegrates. The organism differentiates into a trophozoite that moves freely in the host cell cytoplasm and eventually undergoes binary fission, resulting in two to four merozoites (daughter cells; Fig. 283-1). Merozoites egress the erythrocyte, thereby triggering its lysis, and soon invade other intact erythrocytes.