Fig. 26.1
Split mapping of the drainage of the arm and the breast. SLN and ARM node
The original ARM technique [10, 11] was performed by injecting 1–5 mL of blue dye (isosulfan blue, patent blue or methylene blue) subcutaneously in the inner upper part of the ipsilateral arm. The injection can be made subcutaneously or intradermally although the preferred technique is subcutaneous injection as there are rich lymphatics in this area and the drainage is rapid. Also the time to visualization as well as the tattoo from the blue dye injection is less visible if injected in the upper inner volar surface of the arm versus the hand.
After injection, massage is performed in the area of injection, and the arm is elevated in order to facilitate drainage, similar to the injection of blue dye in the breast for SLNB [10, 11, 14–16]. In the early reports, a time interval of at least 15 min between the injection and surgery was recommended [17], although from other reports, it seems that the arm massage and elevation are more important for drainage than the time interval since the injection [18]. Other authors have not reported improved detection when massaging the arm [14].
In patients undergoing SLNB and an ARM technique, it is crucial that technetium-99 (99Tc) is injected for SLN identification and blue dye for the ARM technique [10]. The SLNB can be performed through a mastectomy incision or an incision in the axilla. The ARM procedure always includes both radioactivity in the breast and blue dye in the arm because, in a small fraction of patients, the ARM node will also be the SLN from the breast [10, 19]. In cases where the SLN is positive and a mastectomy is performed, the ALND can be completed through the same incision; otherwise, a separate axillary incision can be made.
For patients undergoing only ALND, other groups injected patients with 99Tc-labelled nanocolloid alone or combined with blue dye, into the back of the hand on the ipsilateral side or in the upper inner part of the arm [18, 20, 21]. However, it must be emphasized that injecting into the arm only does not allow identification when the ARM node is also draining the breast and would be more likely to contain tumour cells. Split mapping is recommended in all cases.
In those cases where an ALND is performed, an anatomic resection of levels I and II lymph nodes is completed, taking care to identify and preserve blue lymphatics [17–19, 22].
One of the complications of the ARM technique has been the temporary blue tattooing at the injection site. The tattoo fades away but may last from days to years [10, 23, 24] although usually after the first 6 months it is practically invisible [22].
Other authors have used fluorescence imaging technique with subcutaneous injection of indocyanine green (ICG) in the upper inner arm to avoid the blue dye tattoo and to try to improve ARM node identification rates [25–29]. The ICG is injected subdermally into the inner side of the wrist [27] or intradermally in the upper inner ipsilateral arm [26], and the doses used have varied between 0.1 and 1.0 mL [26–28]. In 372 patients, ICG was used combined with blue dye: more than 2 h before surgery, 0.15 mL of ICG was subcutaneously injected into the interdigital area, and immediately before surgery, 1.5 mL of blue dye was subcutaneously injected in the upper third of the arm [28].
The fluorescence signal flowing to the axilla from the upper extremity was detected using a near-infrared fluorescence imaging system as the wavelength is not visible to the naked eye. One of the disadvantages of using an invisible tracer is that the lymphatic channels cannot be followed during surgery and therefore are more likely to be damaged which may increase lymphoedema rates. In the study by Noguchi [29] using a fluorescence imaging system, the SLN was the same as the ARM node in 77 (27%) of 286 patients, and the mean number of removed ARM nodes was 7.2 (range 0–25) in patients who underwent ALND, whereas the mean number of SLNs removed was 1.6 (range 1–7). The clinical importance of the first ARM node or secondary ARM nodes in the lymphatic drainage of the arm is still unknown, but the chances of preserving the ARM node will decrease if the concordance rate between the SLN and the ARM node is higher. However, preserving up to seven nodes while doing an ALND may impact on the oncological safety of the procedure.
26.2.1 ARM Node Location
The lymphatic pathway of the arm is usually described as crossing the lower part of the axilla below the axillary vein, although there is significant variability in reported studies. Most studies have shown that the ARM blue node usually lies in the lateral pillar of the axillary dissection, lateral to the thoracodorsal bundle [14, 25, 30, 31]. The most common pattern identified during SLN is the sling pattern, occurring in 71.4% of the cases as showed by Tummel and colleagues [32] (◘ Fig. 26.2). The ARM lymphatics have been described to be as low as 3–4 cm below the axillary vein, in the medial or lateral apron or even positioned above the vein, although less commonly [19, 22]. The size of the ARM lymphatics is also variable, and they may be as large as 6 mm in diameter [23, 26]. Boneti and colleagues reported that blue lymphatics draining from the arm were visible through the SLNB incision and were located near the field of the SLN in 42% of the 131 patients they reported. This may explain why even with SLNB there are cases of lymphoedema [14].
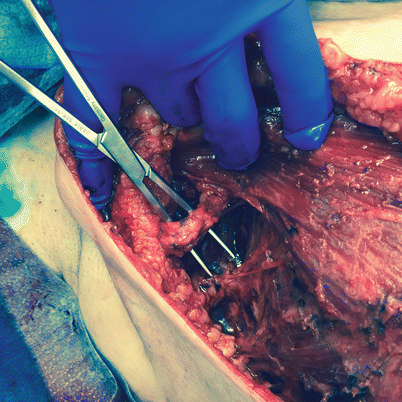

Fig. 26.2
Axillary reverse mapping sling
Regardless of the tracer used for the ARM technique, almost all of the ARM nodes are located in the same area, between the axillary vein and the second intercostobrachial nerve, lateral to the thoracodorsal bundle and close to the latissimus dorsi muscle or in the vicinity.
26.2.2 Identification of ARM Lymphatics and Nodes During SLNB and ALND
Early studies describing the technique showed ARM identification rates of only 61% [10], but with more experience using the technique and increased knowledge of the variations in lymphatics, blue arm drainage identification increased to nearly all patients [31]. Other studies have reported rates of ARM lymphatic and node identification rates of between 27% and 100% [15, 16, 23]. In the meta-analysis by Han and colleagues [33] in eight studies, they found that the pooled results revealed an overall identification rate of 38.2% (95% CI 32.9–43.8%), with statistically significant heterogeneity (I 2 = 70.5%, P < 0.05) [11, 14, 15, 19, 24, 25, 28, 34]. Slight differences in the rate of identification have been shown when using two tracers (78–91%) [11, 21] or using only 99Tc (75–100%) [20, 35]. In this meta-analysis, when they stratified by mapping materials, the pooled identification rate remained similar [33].
Identification rates varied depending on whether an SLN or an ALND was performed. In this way, identification rates in ALND ranged from 70% to 95% in reported studies [18, 20, 21, 25, 26, 28]. The identification rates of ARM nodes have been lower in the SLN biopsy field when compared with those in the ALND field. And this may be explained by the fact that the majority of ARM nodes are located in the upper axilla, whereas the SLNs are located particularly in the lower axilla.
Studies have tried to find if there are variables that impact on ARM node identification rates. Some authors have found that operator experience with the technique improves the identification rate [10, 25], while others have not [17]. Apart from experience with the technique, no statistically significant differences can be observed although in some studies a trend towards a decreased identification rate in patients with a high body mass index [17], following neoadjuvant treatment [22] and in patients with extensive nodal metastases [11, 18, 25, 36], has been observed.
26.2.3 Crossover
Because anatomical studies have shown that the lymphatic drainage from the arm cannot be separated completely from the lymphatic drainage of the breast [9, 13], one would expect to find drainage to the same node from both the arm and the breast in some cases and that has been called crossover, where the ARM node is the SLN. In this situation, the ARM node has to be excised, as it is the SLN, for reasons of oncological safety.
Rates of crossover of the SLN and ARM node have ranged from 0% to 30% [14, 15, 19, 22, 24, 25, 27, 34, 37] in different studies. One important issue to consider is why there is so much difference between studies with some of them showing <5% [14, 19, 32] crossover and others >20% [22, 24, 25, 27, 35]. There are two potential explanations for this: one is variance due to the mapping agent used. The mean number of ARM nodes identified is 7.2 when ICG is used [29], and this may increase the concordance rate, while when the blue dye is used, the mean number of ARM nodes is reduced to between 1.3 and 1.5 [21–23]. On meta-analysis, the crossover rate was 19.6% (95% CI 14.4–26.1%), and when stratified by mapping materials, 8 studies with blue dye showed an overall crossover rate of 7.8% (95% CI 4.2–14.2%), and the only study by fluorescence showed a crossover rate of 28.1% (95% CI 20–37.9%) [33].
The other explanation may be the number of patients enrolled in the study, and in the regression analysis of studies [33], sample size was a significant factor, meaning that the number of enrolled patients may influence the crossover rate of SLN-ARM nodes.
26.3 Metastatic Involvement of ARM Nodes
Involvement of ARM nodes is important, as the objective of the ARM technique is preserving the ARM nodes to achieve lower lymphoedema rates. The issue of the oncological safety of ARM arises as crossover nodes between the arm and breast lymphatics may be found in the axilla and can provide a route for metastatic cancer cells to spread. In order to appreciate the possible role of ARM and subsequent preservation of ARM lymph nodes and corresponding lymphatics when performing axillary surgery for breast cancer, patients may be categorized according to their axillary clinical status.
26.3.1 Involvement of ARM Node in Patients Undergoing SLNB
Rates of involvement of SLN-ARM nodes have been found to be from 0% to 100% [10, 15, 19, 24, 33, 37] (◘ Table 26.1). Only in the study by Deng and colleagues [37] with 69 patients, in 12 patients with positive SLNs, the ARM node was not involved among 50 of 69 patients whose ARM nodes did not coincide with SLN nodes. In the remaining six patients, all metastatic ARM nodes coincided with SLN-ARM nodes. They suggest that perhaps the ARM node involvement only occurs in those patients with crossover, although this has not been proven in other studies. Kuusk and colleagues [24] identified 1 of 5 crossover nodes to be involved, and Ochoa and colleagues [19] found that of the 4% of cases where there was crossover and the SLN-ARM was resected, 2 of 15 nodes (14.3%) were positive. This reflects the fact that in those patients with a positive SLN, where there is no crossover with the ARM nodes, preserving the ARM node may be beneficial. Recent data indicates that one can remove such nodes and re-approximate the afferent and efferent lymphatics with a very low lymphoedema rate as it is thought that these recanalize [32].
Table 26.1
Oncological results of ARM lymph nodes
Study | Inclusion criteria | No. of patient study (No. of procedures) | NAC (%) | Metastatic involvement of the ARM nodes (%) | SLN = ARM (%) |
|---|---|---|---|---|---|
Deng et al. [37] | SLNB | 69 (69) | – | 6/69 (9) | 19/69 (28) |
Rubio et al. [22] | (SLNB + ALND)/CPN+ | 36 (36) | 29/36 (81) | 4/30 (13) | 2/14 (14) |
Casabona et al. [15] | SLNB/SLN+ | 72 (72) | – | 0/3 (0) | N/A |
Boneti et al. [14] | SLNB/SLN+ | 200 (220) | – | 0/15 (0) | 6/214 (3) |
Han et al. [16] | SLNB/SLN+ | 148 (156) | 47/156 (30) | 2/12 (17) | 8/156 (5) |
Boneti et al. [31] | SLNB/SLN+ | 97 (97) | – | 2/17 (12) | 7/97 (7) |
Kuusk et al. [24] | SLNB/CPN+ | 52 (53) | – | 1/11 (9) | 4/52 (8) |
Connor et al. [34] | SLNB/SLN+/CPN+ | 184 (212) | 81/212 (38) | 0/18 (SLNB) (0) 0/4 (SLN+) (0) 4/15 (CPN+) (27) | 22/196 (11) |
Thompson et al. [10] | SLNB/SLN+/CPN+ | 40 (50) | – | 0/7 (0) | 0/46 (0) |
Ochoa et al. [19] | SLNB/SLN+/CPN+ | N/A (360) | – | 5/27 (19) | 15/348 (4) |
Noguchi et al. [27] | SLNB/SLN+/CPN+ | 20 (20) | – | 0/2 (SLN+) (0) 3/5 (CPN+) (60) | 2/14 (14) |
Noguchi et al. [25] | SLNB/SLN+/CPN+ | 131 (131) | 19/131 (15) | 5/42 (SLNB/SLN+) (12) 11/29 (CPN+) (38) | 27/96 (28) |
Ponzone et al. [17] | SLN+ | 49 (49) | 8/49 (16) | 3/27 (11) | N/A |
Bedrosian et al. [23] | SLN+/CPN+ | 30 (30) | 22/30 (73) | 2/15 (13) | N/A |
Gobardhan et al. [38] | SLN+/CPN+ | 93 (93) | 57/93 (61) | 0/37 (SLN+) (0) 11/47 (CPN+) (23) | N/A |
Nos et al. [39] | SLN+/CPN+ | 23 (23) | 11/23 (48) | 3/21 (14) | N/A |
Tausch et al. [18] | SLN+/CPN+ | 143 (143) | 25/143 (17) | 14/55 (24) | N/A |
Ikeda et al. [26] | SLN+/CPN+ | 98 (98) | 20/98 (20) | 17/53 (32) | N/A |
Khandelwal et al. [42] | cT3/cT4/N2–3 | 51 (51) | 51/51 (100) | 0/30 (USG+) (0) 12/15 (USG–) (80) | N/A |
Nos et al. [11] | CPN+ | 21 (21) | 7/21 (33) | 0/10 (0) | N/A |
Beek et al. [40] | CPN+ | 112 (112) | 91/112 (81) | 13/79 (NAC+) (16) 7/19 (NAC–) (37) | N/A |
Beek et al. [48] | CPN+ | 98 (98) | 98/98 (100) | 5/64 (MRI+) (8) 10/34 (MRI–) (29) | N/A |
Yue et al. [21] | CPN+ | 265 (265) | – | 11/129 (9) | N/A |
Tummel et al. [32] | SLNB/SLN+/CPN+ | 654 (685) | – | 2/44 (4.5) | 18/472 (3.8) |