Aspergillus is a ubiquitous organism with an ecological niche in the soil. Most disease is primarily caused by A. fumigatus , A. flavus , A. niger , A. terreus , and A. nidulans, and classification of the genus has been revised multiple times to incorporate newer molecular definitions. A. fumigatus causes approximately 70% to 80% of cases of invasive aspergillosis (IA), but it is difficult to differentiate from the other closely related species based solely on morphology. A. fumigatus is also responsible for most pulmonary disease, whereas isolated sinus disease is often caused by A. niger and A. flavus . Specific determination of the infecting species of Aspergillus is clinically important as there is a variation in therapeutic susceptibility profiles between and within species. Although there is an estimated global incidence of more than 300,000 cases of IA per year, there are significantly more cases of chronic pulmonary aspergillosis (estimated 3,000,000 per year) and allergic Aspergillus disease (many millions per year).
Epidemiology and risk factors
Although not rigorously defined, most experts define a patient group as high risk for IA if the incidence of IA in that group is reported to be 5% to 10% or higher. Following this approach, pediatric populations falling into this high-risk category are those with new-onset or relapsed acute myelogenous leukemia (AML), relapsed acute lymphoblastic leukemia (ALL), and new-onset ALL using aggressive treatment protocols (i.e., high-risk ALL). Other high-risk patients include those with bone marrow failure syndromes (e.g., myelodysplastic syndrome); hematopoietic stem cell transplant (HSCT) recipients and especially those with allogeneic donors; solid organ transplant (SOT) recipients and especially those undergoing lung, heart-lung, or high-risk liver transplant; patients with underlying chronic granulomatous disease (CGD); and patients receiving prolonged courses of corticosteroids or other immune modifiers.
A prospective French study of all three major populations at risk for IA found that time to development of IA after transplantation was similar in different transplantation settings, with 68% of IA after HSCT diagnosed more than 100 days after transplant, and the majority (18 of 27) of SOT recipients with IA diagnosed at least 100 days after transplant. Multivariate analysis showed that factors independently associated with increased risk of death from IA included older age, diagnosis based on positive culture results with two positive galactomannan (GM) assays, and the presence of pleural effusion or central nervous system (CNS) involvement. The case-fatality rate of IA in patients with acute leukemia was 38%, whereas allogeneic HSCT recipients had a staggering 56% overall case-fatality rate.
The Transplant-Associated Infection Surveillance Network (TRANSNET) study sponsored by the Centers for Disease Control and Prevention evaluated largely adult HSCT and SOT recipients from 23 U.S. medical centers (2001 to 2005) and included a total of 642 cases of IA. The 12-month cumulative incidence of IA in all HSCT recipients was 1.6% compared with 0.63% in SOT recipients. Twelve-week all-cause mortality was 57.5% among HSCT recipients and 34.4% among SOT recipients. Multivariable analysis demonstrated that neutropenia, renal insufficiency, hepatic insufficiency, early-onset (<30 days) IA, proven IA, and methylprednisolone use (often for graft-versus-host disease [GVHD]) were independently associated with mortality. Analysis for SOT recipients revealed that hepatic insufficiency, malnutrition, and CNS disease were independently associated with increased risk of death. Among both HSCT and SOT recipients, receipt of voriconazole as part of the initial antifungal therapy was more common among survivors.
The epidemiology and further risk factors specific to HSCT recipients, SOT recipients, and children with malignancy or bone marrow failure syndromes are discussed in more detail in the following sections.
Hematopoietic stem cell transplant
IA was the most common invasive mold disease in a review of approximately 5500 patients who underwent HSCT. Whereas more than 7% of HSCT recipients had mold infections, Aspergillus infections were the most common, followed by Fusarium , mucormycosis, and Scedosporium infections. The incidence of IA in HSCT recipients has ranged from 3% to 7%, but the true incidence is likely dependent on multiple factors, the most important of which is the type of transplantation (allogeneic vs. autologous). In one study of HSCT recipients with IA, the risk of developing the disease was 12.8 times higher among recipients of allogeneic than autologous HSCT. A prospective French study analyzed 424 cases of IA and found an incidence of 0.9% in autologous and 8.1% in allogeneic HSCT patients, respectively, highlighting a consistent finding that IA occurs much more readily in the allogeneic HSCT recipient.
In allogeneic HSCT recipients, three periods of risk for IA occur: (1) neutropenia after the conditioning regimen; (2) exogenous immunosuppression for prevention or treatment of acute GVHD; and (3) exogenous immunosuppression for treatment of chronic GVHD (after day 100 after transplant). The level of allogeneic donor and recipient HLA disparity is the major determinant for GVHD severity and intensity of immunosuppression to control GVHD, which, in turn, is the major predisposing factor for IA during this risk window. There is a well-characterized bimodal distribution of IA in HSCT recipients that correlates with pre-engraftment neutropenia (median of 16 days after transplantation) and the peak of GVHD (median of 96 days after transplantation). Most patients (86%) with autologous transplants were diagnosed with IA while neutropenic, whereas patients with allogeneic transplants were at greatest risk after engraftment or during impairment of cell-mediated immunity owing to cytomegalovirus (CMV) or GVHD.
A subanalysis of the TRANSNET study data focused on only the 875 largely adult HSCT recipients and found IA was the most common (43%) of all invasive fungal diseases, followed by invasive candidiasis (28%). The median time of developing IA after HSCT was 99 days. Of the 80 cases of IA in autologous HSCT, 50% occurred within 1 month after receipt of transplant, whereas in allogeneic HSCT recipients only 22% occurred within 1 month after transplantation. Autologous HSCT recipients had all-cause mortality of 13% at 12 months, whereas allogeneic recipient mortality was higher at 36% at 12 months. The 12-month cumulative incidence for IA in all HSCT recipients was 1.6% compared with 1.1% for invasive candidiasis, and the overall 1-year survival among HSCT recipients with IA was only 25.4%.
Another large database, the Prospective Antifungal Therapy Alliance also found IA most frequent among the largely adult HSCT recipients studied, and approximately 70% of those HSCT recipients with IA were allogeneic transplants. The median time to develop IA after HSCT was similar (82 days), with a diagnosis of a median of 51 days after autologous transplant and 83 days after allogeneic transplantation.
In several HSCT patient risk factor studies, only moderate-to-severe GVHD, steroid prophylaxis for GVHD, or total body irradiation were significant variables in the multivariate analyses. In one study several parameters in the period from HSCT to diagnosis of fungal disease were found to influence survival, each of which was related to the cumulative dose of prednisolone. In the multivariate analysis, there was a relative risk (RR) of 8.78 of death from IA in patients with acute active GVHD (grade II or more) or extensive chronic GVHD combined with a cumulative total prednisolone dose of more than 7 mg/kg in the week before diagnosis.
Solid organ transplant
In SOT recipients, the intensity of immunosuppression to prevent or treat allograft rejection and coinfection with CMV all influence the risk of IA. Data from the TRANSNET database specific to SOT recipients found IA to be the second most common source of invasive fungal disease (19%) behind invasive candidiasis (53%). The median time to onset of IA was 184 days after SOT, with a 1-year cumulative IA incidence of 0.65% and a 12-month survival of 59%. Data on SOT recipients from the Prospective Antifungal Therapy Alliance analysis also found IA (25%) second to invasive candidiasis. IA was most frequently found in lung transplant recipients (60%). IA developed a median of 400 days after any type of SOT; however, this varied by transplant type. The median time from SOT to IA in liver transplantation was 100 days compared with 504 days and 384 days in lung and heart transplant recipients, respectively. Most cases of IA in liver transplant recipients occurred less than 6 months after transplant, whereas 62% of lung transplant recipients with IA developed disease less than 1 year after transplant. This contrasted to a retrospective review of 158 cases of IA in Spanish SOT recipients that found that 57% had early-onset IA (first 3 months after transplantation). The overall incidence of IA in those SOT recipients was 1.4%, including a similar distribution among specific organ transplants with an incidence of 3% (lung), 2.4% (heart), 2% (liver), and 0.2% (kidney). The overall case fatality rate was 77%, with no significant differences between SOT groups. Risk factors for developing early-onset IA included complicated postoperative period, repeated bacterial infections or CMV disease, and renal failure.
In a large prospective mostly adult French study among those SOT recipients with IA, the highest incidence of IA was for heart transplant recipients (4.8%), followed by lung (4.1%), and significantly dropping for liver (0.8%) and kidney (0.3%). Pediatric-specific data on SOT recipients confirmed the highest incidence for all invasive fungal diseases is in pediatric heart-lung and lung recipients, followed by liver and kidney recipients.
Oncology
In patients with malignancy, myelodysplastic syndrome, and other diseases associated with marrow failure (e.g., aplastic anemia), neutropenia is the most important risk factor for IA. Furthermore, assessing the intensity and duration of neutropenia can help the clinician further refine the risk of IA in a patient. In a large case series of IA events, the majority (59%) of patients had a hematologic or solid tumor malignancy with neutropenia as their primary risk factor. Among the remaining 41%, most of the patients had steroid-reliant chronic obstructive pulmonary disease, asthma, or rheumatologic disorders but did not have neutropenia. The clinical presentation of the two groups differed; the latter group was less likely to have typical symptoms of IA and more likely to have frequent intercurrent pneumonia with another microorganism. The case-fatality rates of both patient types were high; nonneutropenic patients had a case-fatality rate of 89% compared with 60% in neutropenic patients.
The risk of IA is estimated to increase from 1% per day after the first 3 weeks of neutropenia to 4% to 5% per day after 5 weeks. Prolonged or marked macrophage dysfunctions that occur as a result of underlying disease and its treatment can also predispose patients to IA. Therefore the risk of infection is higher with advanced underlying disease, transplantation during relapse of malignancy or chemotherapeutic rescue therapy, GVHD, or concurrent infection such as CMV.
In the French prospective study, the majority of patients with IA had a hematologic malignancy and the largest group had acute leukemia (35%), but the second largest group had chronic lymphoproliferative disorders (22%). For those with acute leukemia, IA occurred for 68% during the induction phase of chemotherapy, when cytotoxic agents are generally the most intense, and for 27% during the consolidation phase.
Corticosteroids are also a well-known major risk factor for the development of IA and can suppress the ability of monocytes/macrophages to kill conidia through inhibition of nonoxidative processes and impairment of lysosomal activity. Corticosteroids also inhibit polymorphonuclear neutrophils in their chemotaxis, oxidative bursts, and activity against hyphae. Generally, corticosteroids suppress macrophages, whereas cytotoxic chemotherapy decreases neutrophil number and function.
Pediatric-specific invasive aspergillosis epidemiology
There is little information on the fundamental epidemiology of pediatric IA, and most overall epidemiologic investigations do not offer pediatric-specific analyses. Analysis of a large U.S. pediatric inpatient database found an annual incidence of 0.4% of IA among all immunocompromised children, and the highest incidence of IA was seen in children who had undergone allogeneic HSCT (4.5%) and those with AML (4%), whereas autologous HSCT had a lower incidence of 0.3%. Specifically, the incidence of IA in patients with AML was significantly greater than the incidence in patients with ALL (RR 5.6, 95% confidence interval [CI] 4.6 to 7.0). Lung SOT recipients had the greatest incidence among those pediatric SOT recipients (5%). Comparing mortality in pediatric patients with specific underlying diseases with and without IA revealed the relative risk (RR) of death was increased in CNS tumors (RR 21.6), ALL (RR 14.9), and lymphoma (RR 13.5), showcasing the overall good survival rates of those common pediatric malignancies and the devastating effect of adding IA to reasonably curable underlying pediatric malignancies. The largest pediatric case series was a retrospective multicenter review of 139 children with IA. A. fumigatus was the species most frequently recovered (52.8%), and the majority of the children had a malignancy with or without HSCT. Significant risk factors that affected survival were immunosuppressive therapies and allogeneic HSCT.
The largest international, prospective case series of any invasive mold disease in children (2007 to 2011) included 98 children with IA. Children with IA and those with other types of invasive mold diseases had similar underlying risk factors, except that children with infections caused by non- Aspergillus species were more likely to have received mold-active antifungal agents preceding diagnosis. Among 43 patients who underwent HSCT, 27 (63%) underwent myeloablative conditioning. Of the 36 HSCT recipients who had complete information regarding the timing of diagnosis of invasive mold disease relative to transplantation, 5 (14%) were diagnosed before or on the day of HSCT and 31 (86%) were diagnosed at a median of 168 days following HSCT (interquartile range 25 to 247 days).
Clinical manifestations
Aspergillus species are relatively unique among pathogens as they are responsible for a gamut of infections extending across the clinical spectrum to include primary allergic reactions, saprophytic involvement, chronic disease, and acute invasive disease. The type of Aspergillus infection generally depends on the immunologic background of the infected host, and the focus here is exclusively on immunodeficient patients in whom acute invasive disease develops. The clinical manifestations of these infections in immunocompromised patients can be subtle, nonspecific, and commonly occur late in the course of disease. As a result, a high index of suspicion should be maintained to implement treatment in the early stages of disease.
Invasive pulmonary aspergillosis
Aspergillus species are ubiquitous in the environment and one major portal of entry is the respiratory tract. In some immunocompetent patients, this inhalation could result in nonpathogenic saprophytic colonization; however, in immunocompromised patients, this conidial acquisition will likely result in establishment of invasive disease. Invasive pulmonary aspergillosis (IPA) is the most frequently documented form of IA.
The clinical manifestation of IPA is heterogeneous; typically it may include fever unresponsive to broad-spectrum antibiotics, dry cough, shortness of breath, pleuritic chest pain, hemoptysis, and pulmonary nodules or infiltrates on radiography. Although neutropenic patients more commonly present with fever, in some patients the fever and cough are not present for the first several days of infection, specifically in patients receiving high-dose corticosteroid therapy. Progression of infection is characterized by invasion of small vessels leading to hemoptysis as a leading symptom of IPA in some neutropenic patients. Two patterns of hemorrhage may be identified—hemorrhagic infarction as the result of vascular invasion or formation of mycotic aneurysms during recovery from neutropenia that can rupture and result in fatal hemoptysis.
Invasive Aspergillus sinusitis
Fungal sinusitis can manifest as allergic, saprophytic, or invasive disease. Invasive Aspergillus sinusitis is likely underdiagnosed because of its variable clinical presentation and difficulty in establishing the diagnosis, possibly owing to a decreased inflammatory response in affected patients. Patients can present with nasal congestion, discharge, headache, facial pain or swelling, and abnormal findings of the nasal cavity, such as pallor of the nasal septum or turbinate mucosa. Epistaxis, orbital swelling, and high fever can also be present. However, definitive diagnosis can be established only by endoscopic evaluation and biopsy. Common findings on endoscopy include pallor of the mucosa, discoloration or granulation of the mucosa owing to ischemia as a result of angioinvasion, and as the disease progresses, a blackened necrotic focus can be found. Extension into bony structures can occur at the site of necrosis. leading to spread of disease into adjacent structures, such as the orbit and the brain, which carries high morbidity and mortality. Although imaging is not diagnostic, it can aid in establishing the diagnosis because it can be used as a road map for endoscopy by showing which sinuses are involved. Lack of bony destruction on imaging should not deter pursuit of a diagnosis of IA, as bony destruction is a late manifestation of this process.
Cerebral aspergillosis
IA most commonly involves the lungs, but disease can disseminate via the bloodstream and involve distant organs. One of the most frequent sites of dissemination is the CNS. Cerebral aspergillosis may also be a result of direct extension through the sinuses. As with other Aspergillus infections, A. fumigatus is the most frequently encountered species in cerebral aspergillosis, but other implicated species are A. flavus , A. niger , and A. nidulans . More classical symptoms for an intracranial process, such as headache, nausea, or vomiting, are often absent in cerebral aspergillosis. Instead patients present with mental status alteration, convulsions, hemiplegia or hemiparesis, ophthalmoplegia and loss of consciousness. Severely immunocompromised patients may not display these symptoms and disease progresses more rapidly.
Aspergillus hyphae are angioinvasive and thrombose arteries to create hemorrhagic infarcts, and as a result, CNS aspergillosis can present as solitary or multiple abscesses, and less commonly, as mycotic aneurysms and carotid artery invasion. Cerebral aspergillosis can also appear as meningitis or granuloma. Cerebral aspergillosis presents as multiple areas of low density and no enhancement even with contrast on computed tomography (CT), and lesions are usually located within the basal ganglia and gray-white matter junction. On magnetic resonance imaging (MRI), these same abnormalities appear as foci of intermediate T2 signal surrounded by a rim of higher signal. Aspergillosis of the CNS carries an extremely high case-fatality rate, so prompt diagnosis and treatment are key to survival. Unfortunately, definitive diagnosis requires biopsy and typically these patients are often too coagulopathic to undergo such a procedure.
Cutaneous aspergillosis
Cutaneous aspergillosis can be primary, as is more often seen in children via a result of direct skin injury or traumatic inoculation, or secondary, as a result of hematogenous spread or extension from infected underlying structures. Primary cutaneous aspergillosis has been associated with intravenous access devices, adhesive dressings, and sites of skin compromise such as from GVHD or surgery. Cutaneous disease can also develop from secondary hematogenous seeding from a primary source, usually the lungs. This has been described particularly among HSCT recipients. Lesions often begin as erythematous, indurated papules that progress to ulcerative, painful, and necrotic lesions.
Disease prophylaxis/prevention
Two main strategies exist for managing patients at high risk for IA, primary prophylaxis or no antifungal prophylaxis with close monitoring (generally twice weekly) using biomarkers. The latter is often referred to as a preemptive therapy approach in which a positive fungal biomarker, such as GM antigen or a chest CT scan with pulmonary infiltrates, triggers the use of antifungal treatment while a more confirmatory diagnosis is undertaken. The preferred approach is the source of much debate and likely depends on local epidemiology and the ability to access rapid fungal diagnostics. Notably, there are limited pediatric-specific data on primary prophylaxis or preemptive therapy approaches in children.
In adult guidelines, primary prophylaxis for IA is recommended for patients with hematologic malignancy or those undergoing allogeneic HSCT during periods of neutropenia and at times of GVHD treatment. Posaconazole is recommended as first-line prophylaxis, with other agents such as lipid amphotericin B products, echinocandins, or voriconazole considered as less desirable alternatives. The 2019 ESCMID-ECMM pediatric-specific guidelines for IA state that primary antifungal prophylaxis should be considered during the granulocytopenic phase of allogeneic HSCT. Suggested agents for prophylaxis include itraconazole, posaconazole (for patients ≥13 years), and voriconazole (for patients ≥2 years). Alternative agents include liposomal amphotericin B and micafungin, and less recommended options include aerosolized amphotericin B and caspofungin. In the absence of GVHD, antifungal prophylaxis can continue after engraftment until discontinuation of immunosuppression and signs of immune recovery, but in the presence of GVHD requiring augmented immunosuppression, continuation of antifungal prophylaxis is recommended. These guidelines also recommend IA prophylaxis in children with neutropenia during periods of new-onset or relapsed AML, relapsed ALL, and for bone marrow failure syndromes. In children undergoing SOT, antifungal prophylaxis is recommended in those undergoing lung, heart-lung, heart alone with a high-risk profile, and high-risk liver transplant. It is important to note that there are limited pediatric data from either randomized trials or comparative observational studies on the effectiveness of prophylaxis. As such, most of the aforementioned recommendations for pediatric IA prophylaxis are based on expert opinion.
A preemptive approach with surveillance testing results dictating initiation of antifungal therapy represents an alternative approach to primary prophylaxis. There are no data to compare preemptive and primary prophylaxis approaches in children. However, a randomized trial compared the preemptive versus the empirical antifungal approach (initiation of antifungal therapy after prolonged period of fever and neutropenia) in 149 children with high-risk febrile neutropenia demonstrated that the preemptive approach using molecular biomarkers was associated with similar rates of invasive fungal disease and mortality, and resulted in a significant reduction of antifungal use compared with the empirical therapy approach. Unfortunately, this study does not inform about the effectiveness of this approach compared with primary prophylaxis.
Diagnosis
The diagnosis of IA is not straightforward, and involves integration of clinical, radiologic, and microbiologic data ( Fig. 24.1 ). Because of myriad clinical presentations, IA diagnosis is categorized as “proven,” “probable,” or “possible” disease based on meeting certain clinical, microbiologic, and radiologic criteria designed and later revised by the European Organization for the Research and Treatment of Cancer and the Mycoses Study Group. These criteria have served as a standard in clinical trials and observational studies to segregate patients with similar disease characteristics, but it should be noted that these criteria are not perfect and the designers have specifically cautioned against their implementation in routine clinical practice. Nonetheless, these distinctions have served the community well to establish a common framework for discussion about disease in the complicated high-risk patient. Generally, “proven” and “probable” IA can be considered as one entity, as numerous clinical trials have shown their general equivalency in patient outcomes.
Cultures
A proven diagnosis of IA requires isolation of an Aspergillus spp. from a culture specimen taken from an otherwise sterile site in association with histologic evidence of invasive disease. Although the histopathologic appearance of hyphae can provide a proven invasive fungal disease [IFD] designation, confirmation of IFD as IA requires detection of Aspergillus by culture or nonculture technique. This is necessary because Aspergillus cannot be distinguished histopathologically from other filamentous fungi, such as Fusarium spp. and Scedosporium spp.
Unfortunately, the requirements for proven IA diagnosis are challenging to meet, especially because biopsy specimen procurement is often considered too invasive and is complicated by bleeding or secondary infection in high-risk patients. Detection of Aspergillus in noninvasive specimens, such as sputum, allows for designation of probable IA in association with radiographic findings, but sputum culture is complicated by the fact that the presence of Aspergillus may represent colonization and not disease. The sensitivity of sputum culture for Apsergillus is poor likely because IPA is predominantly infiltrative and does not have aerial growth in the bronchial tree. In one study of heart transplant recipients, during a 10-year study period, Aspergillus species were recovered from 30 episodes from 27 heart transplant recipients (incidence 10.5%). The overall positive predictive value was 60% to 70%, but this increased to 88% to 100% when it was recovered from a respiratory specimen other than sputum, and decreased to 50% to 67% when it was recovered from sputum. Taking all of this into consideration, it is likely that the incidence of IA is underestimated in many published cohorts.
Isolation of Aspergillus spp. from the blood is difficult, and thus any positive blood culture result for Aspergillus spp. is often presumed to be a laboratory contaminant and not a true infection. The difficulty in detecting A. fumigatus in blood culture stands in contrast to other angioinvasive filamentous fungi (e.g., Fusarium spp., Paecilomyces lilacinus, Scedosporium prolificans, Acremonium spp.) that have the ability to discharge a steady series of unicellular spores into the bloodstream, which are more likely to be captured in a blood sample. This ability to sporulate in tissue and blood has been termed adventitious sporulation. As A. terreus also displays adventitious sporulation, a positive blood culture with A. terreus or another mold that demonstrates adventitious sporulation should not be ignored.
Radiology
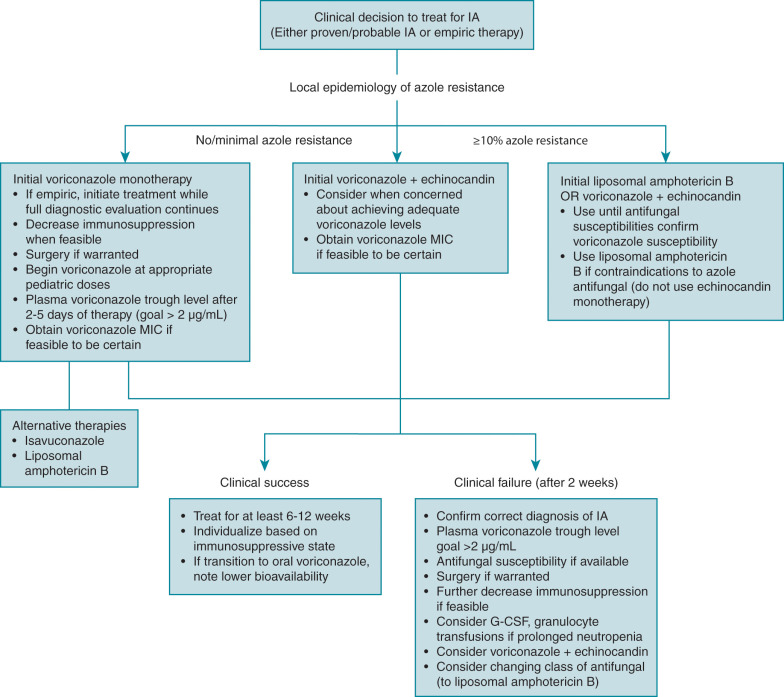
In high-risk patients when there is concern for IFD (e.g., in a child with persistent fever and neutropenia despite broad-spectrum antibacterial treatment), further investigation with radiographic imaging is warranted. The focus of this section is on the radiographic findings of the lungs, brain, and sinuses, as these are the most likely sites of IA. However, Aspergillus can disseminate hematogenously to any location of the body from one of these primary sites. Therefore imaging of the abdomen or musculoskeletal system may be warranted in some settings. High-resolution CT scan is considered the imaging modality of choice for the lungs and sinuses, whereas MRI is preferred for evaluation of the brain.
Pulmonary imaging.
IPA characteristically manifests as multiple, ill-defined, 1- to 3-cm peripheral nodules that gradually coalesce into larger masses or areas of subsegmental and segmental consolidation. Lobar, pleural-based wedge-shaped, alveolar, or diffuse pulmonary consolidation are also common findings. , Plain chest radiographs can detect some of these pathologies, but they are insensitive and can result “normal” in patients even in the week preceding death. The aforementioned findings are relatively nonspecific findings; however, there are two radiologic signs—the halo sign and the air crescent sign—that were previously thought to be considered highly suggestive of IPA. The halo sign occurs in neutropenic patients with a hemorrhagic nodule owing to angioinvasion. An early CT finding of the halo sign is a rim of ground-glass attenuation opacity surrounding the nodule. These early lesions subsequently change into a cavitary lesion or lesion with an air crescent sign 2 to 3 weeks later when neutropenia recovers. Cavitation of the nodules or masses occurs in about 40% of patients and is characterized by an intracavitary mass composed of sloughed lung and a surrounding rim of air. Although the halo sign can be seen in patients with biopsy-proven IPA, it is nonspecific and can be seen in patients with mucormycosis, organizing pneumonia, or pulmonary hemorrhage.
A long-term CT follow-up in 40 immunocompromised patients with IPA showed that formation of cavitation most strongly predicted time until radiologic remission and beneficial outcome. In that study, the natural history of early IPA lesions was evaluated and it was found that 90% of patients experienced an increase in lesion size and number followed by a plateau in size and a decrease in number. Cavitation of the lesions developed in 55% of patients and complete radiologic remission, within a median 80 days, was observed in 42.5% of patients. The number of days until remission without cavitation (50 days) was less than for those with cavitation (95 days), so formation of no cavitation was strongly predictive of radiologic remission. Repetition of a CT scan before 2 weeks after the start of treatment is not usually recommended unless the patient experiences clinical deterioration. An exception is the presence of a nodule close to a large vessel because of the risk for massive hemoptysis if lesions continue to increase in size. Routine use of contrast with CT is not recommended.
There may be radiologic differences for IA in adult and pediatric patients. In adult series of IPA, approximately 50% of cases show cavitation and 40% air crescent formation. In one 10-year review of pediatric patients (mean age 5 years), there was central cavitation of small nodules in only 25% of children and no evidence of air crescent formation within any area of consolidation. The largest series of contemporary pediatric IA cases found the most common radiologic finding for IPA was nodules (34.6%). Importantly, only 2.2% of the children showed the air crescent sign, 11% demonstrated the halo sign, and cavitation was seen in 24.5% of patients. Other pediatric series with higher mean ages have slightly higher rates of cavitation and air crescent formation, suggesting that there may be a spectrum of radiologic disease presentation that is directly related to age, with cavitation and air crescent formation more likely in the older child and adult than in the younger child.
A review of serial radiographs of 27 HSCT pediatric recipients with IFD highlights how radiographic findings can change over time. Although this study was not exclusive to IPA, it highlighted the wide range of possible radiographic findings on lung imaging and the change in appearance over time. At initial detection, unilateral infiltrates (52%) were slightly more common than bilateral infiltrates, and the infiltrates were interstitial (41%), alveolar (41%), and mixed (18%). Hilar or mediastinal lymphadenopathy and pleural effusion/thickening were rare. On follow-up, the infiltrates were more commonly bilateral (66%) and alveolar or nodular (74%), including 22% of patients who had cavitary lesions.
In selected patients in whom CT is not feasible, thoracic MRI is an alternative but findings are not as characteristic as chest CT findings, and the typical MRI sign is the target sign, a nodular lesion with a lower signal in the center compared with a higher, contrast-enhancing signal intensity in the rim on T1-weighted images.
Brain imaging.
MRI is the modality of choice for diagnosing cerebral aspergillosis and is preferred over CT for sensitivity. Findings often show multiple lesions located in the basal ganglia that include an intermediate signal intensity, lack of contrast enhancement, and absence of mass effect. Although MRI is preferred for its ability to more completely assess the brain, CT of the head can still be useful, especially if an MRI is not attainable. The head CT scan often reveals one or multiple hypodense, well-demarcated lesions. Hemorrhage and mass effect are unusual, but for patients with adequate peripheral white blood cell counts a ring enhancement and surrounding edema are more frequent.
Sinus imaging.
Although imaging is not diagnostic, it can aid in establishing the diagnosis because it can be used as a road map for endoscopy by showing which sinuses are involved. In a review of 25 patients with rhinosinusitis, 44% showed evidence of invasion beyond the sinus cavities on CT scan. In the same study, however, 12% of patients had negative CT scan results, again highlighting that a high index of clinical suspicion must guide establishment of the diagnosis to ensure the best outcome.
Galactomannan antigen
GM is a major cell wall component of Aspergillus . An enzyme-linked immunosorbent assay (ELISA) technique was developed using a rat anti-GM monoclonal antibody, EB-A2, which recognizes the 1→5-β-D-galactofuranoside side chains of the GM molecule. A sandwich ELISA technique was introduced in 1995 and by using the same antibody as both a capture and detector antibody in the sandwich ELISA (Platelia Aspergillus , Bio-Rad, Hercules, CA) the threshold for detection was lowered to 1 ng/mL. This technique is used in the currently commercially available GM assay for diagnosis of IA.
Serum galactomannan testing.
The latest U.S. guidelines support serum GM as an accurate marker for the diagnosis of IA in adult and pediatric patients when used in certain patient subpopulations (hematologic malignancy, HSCT). This recommendation includes serial screening in patients with prolonged neutropenia not receiving mold-active prophylaxis and is founded on predominantly adult data showing a high sensitivity and negative predictive value for IA in this clinical setting. More recent pediatric data, however, suggest that surveillance testing in pediatric patients is not as useful as it is in adults even in the absence of mold prophylaxis. In adult populations, serum GM is advocated to be sampled twice weekly (every 3 to 4 days), with the highest test accuracy requiring two consecutive positive samples (optical density index [ODI] ≥ 0.5) or retesting the same sample. Currently, pediatric-specific data do not support this broad of an approach.
It is well accepted that routine serum GM screening should not be performed in patients receiving mold-active antifungal therapy or prophylaxis owing to the low prevalence of IA in this setting with a consequently low positive predictive value of the serum GM assay.
Neutropenia also affects GM utility, and the sensitivity of serum GM is significantly lower in nonneutropenic versus neutropenic patients. GM values in adult patients with an absolute neutrophil count (ANC) less than 100 cells/μL and not receiving antifungal therapy were statistically higher than those patients with an ANC greater than 100 cells/μL. However, GM values were not statistically different in patients with an ANC less than 100 cells/μL and receiving antifungal therapy versus those with an ANC greater than 100 cells/μL. It is possible that this neutropenia effect is due to fungal burden higher at the time of initial IA in neutropenia or that IA lesions are more extensive and possibly result in angioinvasion in the setting of neutropenia.
The specific patient population tested is critical to optimizing GM utility. Although GM has been extensively validated in patients with hematologic malignancy and those who have undergone HSCT, for unclear reasons GM appears less useful in SOT recipients. The U.S. guidelines do not recommend GM for screening in SOT recipients because of the low predictive value and high false-negative rates, respectively. In liver transplant recipients, there was a high false positivity, especially in patients with autoimmune liver disease or dialysis, and in lung transplant recipients, there was a greatly decreased sensitivity, perhaps owing to the pathophysiologic differences in Aspergillus tracheobronchitis or anastomotic disease seen in these patients.
False-positive results with GM can hamper its clinical utility and are seen in patients concurrently receiving some β-lactam antibacterials. Importantly, piperacillin-tazobactam, once the major cause of this false-positive reaction, is now no longer cross-reactive. Several years ago, there were concerns of a supposed increased false positivity in children, and one theory suggested it was due to (1) Bifidobacterium bifidum spp. in the gut microflora, which mimics the epitope recognized by the EB-A2 in the ELISA kit, and (2) GM-positive infant formula used in pediatric patients. False positivity is likely due to the ELISA testing itself cross-reacting with specific antigens, likely not all of which have been defined, and this phenomenon appears irrespective of the patient’s age.
Diagnosis of pediatric IA with GM, although originally reported to be less useful owing to a higher false-positive rate, has been validated to also be effective in children when used in the correct patient population. Multiple pediatric GM studies for focused testing, not surveillance, were reviewed to highlight the current knowledge of this assay in children, and it was determined that the GM assay has similar operating characteristics in pediatric as well as in adult patients.
Bronchoalveolar lavage.
Guidelines state that bronchoscopy with bronchoalveolar lavage (BAL) is recommended in patients with a suspicion of IPA. The yield of BAL is low for peripheral nodular lesions, so that percutaneous or endobronchial lung biopsy should be considered. BAL should include routine culture and cytology, and most importantly non–culture-based methods (e.g., GM), as routine culture alone can have a low sensitivity. BAL is often useful in diagnosing IA, but a negative BAL culture result does not conclusively rule out disease.
The ability to detect Aspergillus in BAL samples may be increased by use of GM, thus increasing the yield of bronchoscopy and possibly avoiding the need for further invasive procedures. Although the GM cutoff value in sera is 0.5, the cutoff is best used at 1.0 for BAL GM to increase the accuracy of the test. A retrospective analysis of 99 high-risk hematology patients, including 58 with IA, who underwent BAL for the diagnosis of new pulmonary infiltrates found that a BAL GM value of 1.0 or higher yielded an increased sensitivity (91.3%) compared with BAL culture (50%) or BAL microscopy (53.3%). The combined sensitivity of all three BAL methodologies was 98.2%. Further analyses in that study found that the mean BAL GM value was not different in neutropenic versus nonneutropenic patients.
A meta-analysis of BAL GM studies found the sensitivities between 59% and 100% and specificities between 76% and 100%, and most importantly, that antifungal therapy did not significantly affect sensitivity as it does with serum GM. A retrospective study in hematologic malignancy patients and HSCT recipients also found that BAL GM was more sensitive than serum GM for diagnosing IA, and found that BAL GM was not affected by mold-active antifungals, suggesting that this may be due to the levels of antifungals found in sera versus alveolar fluid. A retrospective pediatric BAL GM found an optimal BAL GM cutoff value of 0.98 to yield the best sensitivity (78%) and specificity (92%). Using a BAL GM value of 1.0 or higher and a concurrent serum GM value of 0.5 or higher yielded the best sensitivity (89%) and specificity (90%).
(1,3)-β-D-Glucan
(1,3)-β-D-Glucan is an integral cell wall component and, in contrast to GM, is not normally released from the fungal cell. Factor G, a coagulation factor of the horseshoe crab, is a highly sensitive natural detector of (1,3)-β-D-glucan. The G test detects (1,3)-β-D-glucan via a modified limulus endotoxin assay but does not identify the genus of the fungi detected. Unlike the GM assay, this assay is nonspecific as (1,3)-β-D-glucan is present in several different fungi, including Aspergillus spp., Candida spp., Fusarium spp., Trichosporon spp., Saccharomyces cerevisiae, Acremonium, Coccidioides immitis, Histoplasma capsulatum, Sporothrix schenckii , and Pneumocystis jirovecii . The (1,3)-β-D-glucan assay does not detect Cryptococcus and the yeast form of Blastomyces dermatididis (which produce low levels of (1,3)-β-D-glucan) or organisms from the Mucorales Order (which produce no (1,3)-β-D-glucan). Importantly, this assay does not identify the genus of the fungi detected, only the presence of the fungal call wall component.
False-positive results can occur in a variety of contexts, such as through glucan-contaminated blood collection tubes, gauze, depth-type membrane filters for blood processing, intravenous immunoglobulin, and various drugs (e.g., antibiotics, including some cephalosporins, carbapenems, and ampicillin-sulbactam and possibly chemotherapeutics such as pegylated asparaginase). The Fungitell assay (Associates of Cape Cod, East Falmouth, MA) for detection of (1,3)-β-D-glucan is cleared by the U.S. Food and Drug Administration for the diagnosis of invasive mycoses, IA, and has been evaluated in high-risk patients with hematologic malignancy and allogeneic HSCT.
In one study comparing (1,3)-β-D-glucan and GM, the sensitivity, specificity, and positive and negative predictive values for GM and (1,3)-β-D-glucan were identical. False-positive reactions occurred at a rate of 10.3% in both tests, but the patients with false-positive results were different in each test. Both tests anticipated the clinical diagnosis and CT abnormalities, but the (1,3)-β-D-glucan result tended to become positive earlier than GM. A combination of the two tests improved the specificity (to 100%) and positive predictive value (to 100%) of each individual test without affecting the sensitivity and negative predictive values. A meta-analysis of cohort studies of (1,3)-β-D-glucan for IA revealed that using a single test resulted in a pooled sensitivity of 57% with a specificity of 97%.
Recent guidelines state serum assays for (1,3)-β-D-glucan are recommended for diagnosing IA in high-risk patients (hematologic malignancy, allogeneic HSCT) but are not specific for Aspergillus. Current guidelines for children recommend against (1,3)-β-D-glucan testing for screening or evaluation of suspected IA because of the limited data in children and the unknown optimal cutoff value in pediatric patients. , ,
Polymerase chain reaction
The exact clinical utility of blood-based polymerase chain reaction (PCR) in diagnosing IA is currently unclear, and experts debate the present utility for standard clinical practice of this diagnostic modality until conclusive validation of standardized commercially available assays. Recent guidelines state that direct comparison studies have shown Aspergillus PCR to be substantially more sensitive than culture. In a meta-analysis of clinical trials evaluating the accuracy of serum or whole-blood PCR assays for IA for various indications, sensitivity and specificity were 84% and 76%, respectively. These values are promising, but PCR of blood or serum is unable on its own to confirm or exclude suspected IA in high-risk patients. The sensitivity of Aspergillus PCR on BAL fluid was higher than within blood, but in many instances its specificity was lower. In a systematic review of nine studies using reference IA definitions strictly adherent to the European Organization for the Research and Treatment of Cancer and the Mycoses Study Group criteria, the sensitivity and specificity of PCR of BAL were 77% and 94%, respectively. The lower specificity in BAL has been attributed to the fact that lungs are often colonized by Aspergillus (particularly in many high-risk populations, such as lung transplant recipients), and that PCR is not able to differentiate colonization from disease or to distinguish different Aspergillus spp. The high negative predictive value of BAL PCR (usually ≥95%) suggests a role in ruling out IPA. To date, data suggest that the diagnostic performance of blood or BAL PCR is comparable to that of serum and BAL GM index and that sensitivity for both tests is affected by antifungal use. Using both PCR and GM in serum resulted in improved sensitivity with no sacrifice of specificity.
Despite these promising results, Aspergillus PCR cannot yet be recommended for routine use in clinical practice because few assays have been standardized and validated, and the role of PCR testing in patient management is not established. Initiatives such as the European Aspergillus PCR Initiative have made significant progress in developing a consensus standard protocol for blood-based Aspergillus PCR. At present, this diagnostic method is not commercially available, and reports can be difficult to interpret because of the lack of experimental standardization between centers. Owing to the ubiquitous nature of the mold, it is likely that the value of this test will be its high negative predictive value. Because of the lack of sufficient pediatric data, there is no guideline recommendation for PCR in the diagnosis of IA for children. , ,
Treatment
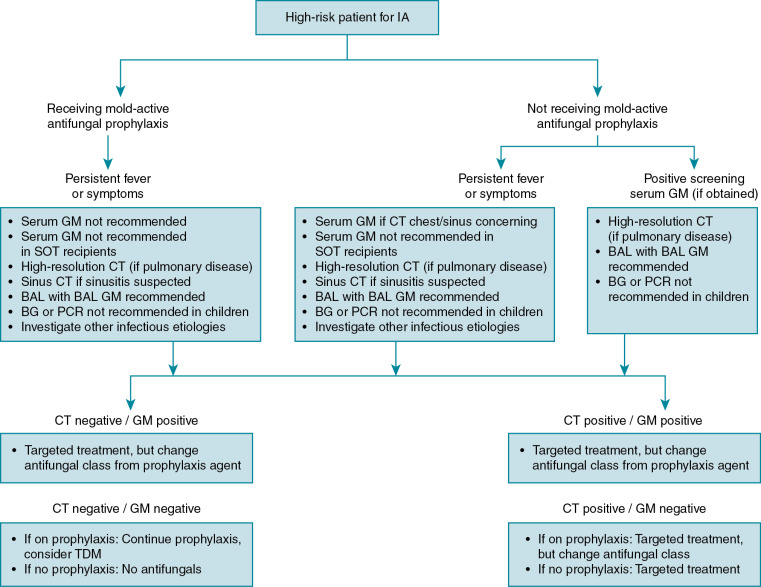
Overall success in treating IA is dependent on numerous factors, not simply the choice of a specific antifungal therapy ( Fig. 24.2 ). As with all immunocompromised patients, detailed knowledge of host factors, underlying disease, concomitant infections, and the degree and duration of immunosuppression are key to overall management. It is well known that immune reconstitution is paramount to successful IA therapy, and continued exposure to certain immunosuppressive medications, such as corticosteroids, is known to worsen IA. Any antifungal prophylaxis used before the diagnosis of IA could also have an effect on the ultimate choice of empiric or targeted therapy. The diagnostic workup needs to be aggressive to confirm disease, but it should never delay antifungal therapy in the setting of true concern for IA. The cornerstone of antifungal therapy for IA is prompt and aggressive institution of antifungal therapy, based not only on diagnostic results but also on clinical suspicion of infection if diagnosis is not immediate. Antifungal resistance is now slowly increasing among Aspergillus isolates and continues to have specific geographic trends that could influence antifungal choice. For the moment, resistance among Aspergillus isolates isolated from patients in the United States is rare. There is also the question of using antifungal monotherapy or combination antifungal therapy, and if so, which classes of agents. Finally, although immune reconstitution is paramount, the role and real benefit of adjunctive immunotherapy remains somewhat unclear. Treatment for most forms of IA follows the recommendations made for the more common invasive pulmonary aspergillosis.