Fig. 1
Macroscopic findings: the origin of a thrombus in the popliteal vein (arrow)
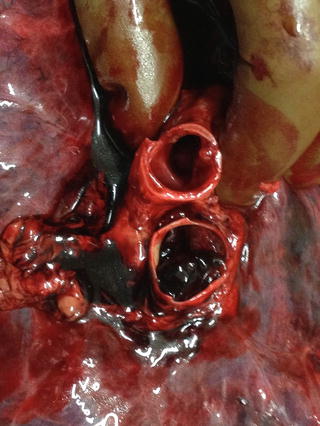
Fig. 2
Macroscopic findings: pulmonary embolism
Usually, the starting point of emboli are leg deep veins, but autopsy operators must keep in minds that also other sites can be involved. For example, it is therefore important to examine the pelvic venous plexuses for thrombi, even if demonstration of pelvic vein thrombosis as the source of pulmonary thromboemboli is rare. Periprostatic or periuterine venous thrombosis are reported in literature. Of course, the diagnosis of thrombosis in these sites is most likely to be made only during postmortem examination. Other sites of clot origin may be suspected based on the clinical presentation (for example the atrial appendage of the right heart or the vessel wall at the tip of a venous catheter). Despite all, there is a group of patients for whom the site of clot origin is an enigma. The autopsy operator must keep in mind the pathways for emboli migration. These pathways may be, for example, the inferior vena cava or Batson’s plexus through paravertebral veins (Elhammady et al. 2011).
Other autopsy finds that must be considered in the suspect of pulmonary embolism are cardiac and pulmonary findings.
Heart PTE-related mortality is considered to have a strong relationship with right ventricular dysfunction. As morphological changes with acute right ventricle failure due to volume overload, an enlargement of right ventricle was found in almost all the cases of PTE. In addition to the acute changes, right ventricular hypertrophy caused by continuous pulmonary hypertension was concomitantly observed in the most part of these subjects. These findings suggested that the patients had few subjective symptoms from the latent history of recurrent thromboemboli, and that the previous submassive PTE easily induced a pulmonary circulation disturbance resulting in right ventricular hypertrophy.
At the end, the most specific finding related to acute PTE at autopsy is the presence of thromboemboli in main pulmonary artery, which are easily found in routine autopsies with fatal PTE. Sometimes, it is possible to notice the marks of the venous valves that certificated the embolic sources. These emboli can be white or black emboli. Black emboli are easily found at the main pulmonary artery or at the segmental artery, while the white ones are usually found at the subsegmental artery or at the small elastic artery. White emboli are the remnants of fresh thrombi changed by the organizing process. These organized thrombi are useful for indication of latent past thromboemboli in patients subsequently examined in the forensic autopsy. However, detailed autopsies of cases of fresh massive thromboemboli could detect also organized thrombi in the same patient: Morpurgo and Schmid (1980) stated that 15 % of the PTE patients showed multiple infarctions occurring at different ages. Macroscopically, the more distal site at the pulmonary parenchyma appeared pale due to pulmonary circulatory ischemia. However, pulmonary infarction is an infrequent finding, because it occurs only in the 21 % of the cases with massive PTE. This can be due to dual perfusion of the pulmonary artery and the bronchial artery.
3 Microscopic Examination of Pulmonary Thromboembolism
Generally, pathologic features of thromboemboli must be estimated using histological sections stained by haematoxylin–eosin (H&E), trichromic stains (Masson, Azan, Mallory, PTAH, Van Gieson), Von Kossa for calcium salts and Perl’s stain for hemosiderin to confirm the presence of iron (Fineschi et al. 2012). All these stains are fundamental for dating the embolic event. In fact, three phases of organization of the thromboembolus are known. The first phase (1st–7th day) starts with flowing blood on an eroded endothelium, this condition causes a platelet plug with deposition of fibrin in a layered fashion (Zahn’s lines). In this phase, the thrombus is firmly attached to the vessel and there are not reactions between endothelium and thrombus; erythrocytes are preserved and agglomerated. It is possible to see initial pyknosis of white blood cells and also monocytes cells with enlarged nuclei. Precipitates of Calcium are observed in Von Kossa stain. After the first phase, the second one is characterized by endothelial budding and proliferative changes of the medial ring represented by penetration of fibroblasts. This phase usually lasts from 2nd to 8th week, and it is characterized by a predomination of macrophages containing hemosiderin and fibrinous transformation. The ribbons of fibrin change to a clot of white cells. The surface of the thrombus is covered by endothelium and scattered nuclear debris of white blood cells are observed. The third and last phase, from the eighth week, is characterized by a completely hyalinized thrombus with central sinuous cavities. Lately (older than 2 months), neo-formed larger vessels with fresh flowing blood are observed (Fineschi et al. 2009). As time goes by, if the subject survives, the thrombus become “organized”. Organized thrombi are constituted by eccentric collagen or elastin elements that completely replace erythrocytes and fibrin, the main components of acute thromboemboli. The discovery of organized thrombi during histo-pathological examination, especially in acute PE cases, suggests the previous occurrence of PE, and it is suggested by some authors that the frequency of organized thrombi might reflect the severity of recurrent PE (Ro et al. 2011). These histopathological advice provide very important suggestive information for physicians to predict acute PE at a preventable stage. Many studies demonstrated the frequency of recurrent PE. Dunnill (1987) stated that careful autopsy examination of patients with acute PE usually reveals small organized thrombi. Morpurgo (Morpurgo and Schmid 1980) reported that autopsy findings of multiple PE and pulmonary infarction are obtained in at least 15 % of PE cases (Ro et al. 2011), so further studies are needed to better understand the pathology of PE and discovery new diagnostic tool to monitor these patients which show high risk of thromboembolic events (Freiman et al. 1965).
Regarding the localization, it was demonstrated that fresh thromboemboli are more frequently located in the right lung than in the left one, and furthermore the lower lobes are mostly involved (Wagenvoort 1995). This is related to the flow distribution, which favors the right lung and the lower lobes. The highest frequency of organized thrombi is in the right pulmonary artery in the posterior basal lobe, even if it is possible to detect organized thrombi in the upper and middle lobes (Moser and Bloor 1993). Another aspect that autopsy operator must consider is that thromboemboli are usually trapped and organized at the bifurcation of the elastic artery (Morrell and Dunnill 1967), and that organized thrombi in the muscular artery arise as a consequence of fragmentation of previous PEs lodged in the proximal elastic artery.
To conclude, our message is to underline that autopsy confirmation of thrombi is fundamental for estimating the subclinical history of patients with recurrent PE, and it has an inestimable value for the diagnostic classification of family members of the deceased subject and to prevent other deaths.
4 Immunoistochemestry of Pulmonary Thromboembolism
As discussed above, PTE is an important disease for legal medicine for several reasons: it can be necessary to assess the causal relationship between PTE and recent accident, such as trauma, or it can be necessary to judge the medical practice in the cases of in-hospital PTE among patients who were treated for other diseases, or it can be necessary to investigate the pathology of PTE as a cause of sudden cardiovascular death. It is important, in a medico-legal contest, to know if a pulmonary embolus arose prior to, or subsequent to, some traumatic event. A major difficulty is that the embolus may be the most recent addition to an extending, older venous thrombosis. The best method to evaluate this condition is to examine and to try to date the residual thrombus (Fineschi et al. 2009). Historically, for the first time Iringer et al. (Irninger 1963) proposed histological aspects about forensic-histological age determination of thrombi. Subsequently, Leu et al. (Leu and Leu 1989) established representative findings in relation with thrombotic changes and chronological organization. In the last years, many interest are arising about the application of immunoistochemestry with the purpose to date the thrombus, because immunohistochemical markers are more sensitive and specific than conventional markers.
Immunohistochemistry (IHC) is a technique that combines anatomical, immunological and biochemical methods to identify discrete tissue components by the interaction of target antigens with specific antibodies tagged with a visible label. IHC visualize the distribution and localization of specific cellular components within cells and in the proper tissue context. A limitation of this method is that it is routinely used with tissues after fixation, and fixation chemically crosslinks proteins or reduces their solubility, and so it can mask target antigens during prolonged or improper fixation. The most common fixative is formaldehyde, a reagent that can be used for immersion fixation for any length of time, depending on the level of fixation required. Fixed tissue samples are embedded in paraffin to maintain the natural architecture of the sample during long-term storage and sectioning for IHC. Formalin-fixed, paraffin-embedded tissues are usually sectioned into slices as thin as 4–5 μm for IHC diagnostic purpose.
Some studies (Fineschi et al. 2009, 2012; Nosaka et al. 2010, 2015) show that immunohistochemistry permits an objective age determination of the thrombus: the immunohistochemical detection of neutrophils and macrophages in venous thrombi could give significant information for age determination of venous thrombi. Furthermore, the dynamics of other types of cells and extracellular matrix, intrathrombotic collagen contents, and the appearance of hemosiderin-positive cells, myofibroblasts and neovessels are essentially involved in the formation and resolution of venous thrombi. Using histochemical and immunohistochemical techniques, it is possible to investigate these aspects in order to estabilish the thrombus age. Fineschi et al. (2009), for example, proposed immunohistochemical investigation of thrombus and embolus using polyclonal anti-fibrinogen antibodies, CD61, CD45, CD15, CD68. Nosaka et al. (2010), in their studies, evaluated intrathrombotic appearance of hemosiderin-positive cells as useful tool for thrombus age determination: in fact, macrophage recruitment is essential for thrombotic formation. Recruited macrophages can produce various cytokines and phagocytose red blood cells, and hemoglobin is converted into hemosiderin, that can be detected by Berlin blue staining. Hemosiderin- positive cells, myofibroblasts and neovessels were evaluated in these studies: hemosiderin-positive cells constantly appeared later than 5 days, while both myofibroblasts and neovessels were routinely detected at 10 days, demonstrating that these markers may be applicable for thrombus age determination (Nosaka et al. 2010). More recently, the same group showed that the immunohistochemical detection of cytokines and chemokines should be useful for determination of thrombus age. In fact, various kinds of bioactive molecules, such as cytokines and growth factors, are closely involved in the processes of formation and resolution of thrombi. They demonstrated the pathophysiological roles of cytokines such as IL-1, IL-6, TNFα, and IFNγ in the processes of thrombus resolution using genetically engineered mice. Particularly, IL-6 may be a key molecule in the formation and resolution of venous thrombi: thrombus resolution seem to be delayed in the absence of IL-6 through reduced matrix metalloproteinases (MMPs). IL-6 is a pleiotropic cytokine produced by many cells, including macrophages, T cells, fibroblasts, keratinocytes, and endothelial cells, and it seems to have detrimental roles in the thrombus resolution by suppressing intrathrombotic proteinases. However, further studies are necessary to confirm that immunohistochemical detection of intrathrombotic IL-6 would be applicable for thrombus age estimation (Nosaka et al. 2015).
To summarize, we state that the histological age determination of thromboses is an important task of PE and requires through knowledge of the general and specific pathology of VTE. Although further studies are needed, we believe that immunohistochemical study in venous thrombi could give information to determine the age of venous thrombi. However, the observed transformation of the thrombus by organization is suitable for a pathologically utilizable age determination. Furthermore, modern technologies are a valid aid to date the DVT phenomenon and the chronological changes of the thrombus.
5 New Postmortem Diagnostic Tools in Pulmonary Thromboembolism
In the last years, the detecting rate of venous thrombi in the cases of PTE has been increasing both in clinical and forensic medicine, due to the fact that the crural calf vein, including the intramuscular veins, can be more easily visualized by magnetic resonance imaging or ultrasonography than with the previous diagnostic techniques such as venography or computed tomography (Ro et al. 2011). These fundamental diagnostic tools can be performed after a lethal PE, for an useful diagnostic aid in the evaluation of these patients, especially when there are problems to obtain permission for autopsy. Dedouit et al., for example, reported a case in which permission for autopsy was not obtained, but post-mortem computed tomography investigation was performed with the agreement of the patient’s family in order to successfully ascertain the cause of death (Dedouit et al. 2006).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree