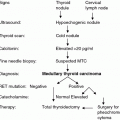
Fig. 1
Organization of the calcitonin/calcitonin gene-related peptide gene and its peptide hormone products. CTN calcitonin, CGRP calcitonin gene-related peptide, KC katacalcin, SP signal peptide, K lysine, R arginine, G glycine, and aa amino acid
Synthesis and secretion of calcitonin are tightly regulated: An almost linear correlation between calcium concentration and the amount of secreted calcitonin could be displayed in a porcine model (Garrett et al. 1995). In particular, the intracellular calcium concentration in the C-cells regulates the secretion rate of calcitonin possibly through the calcium-sensitive receptor, which is also expressed in the thyroidal C-cells. Glucocorticoids, CGRP, glucagon, gastrin, pentagastrin (PG), pancreozymin as well as β-adrenergic agents are also capable of inducing marked increases of calcitonin secretion (Care 1992). On the other hand, somatostatin, a hypothalamic hormone, which can be also secreted by the C-cells, inhibits calcitonin secretion. Calcitriol (1,25–OH–Vitamin D3), another player in the field of calcium homeostasis, may negatively influence the secretion of calcitonin by decreasing the amount of calcitonin mRNA.
It has been shown that calcitonin exerts its effects through the calcitonin receptor—a serpentine protein expressed at high levels in kidney and hypothalamus. In bone, CTN almost exclusively bounds to osteoclasts (Marx et al. 1972). In animal studies, numerous effects of calcitonin on calcium and bone metabolism could be demonstrated. For instance, calcitonin lowers tubular reabsorption of calcium when acutely administered (Friedmann and Gesek 1995). Furthermore, it impairs osteoclast-mediated bone resorption (Chambers et al. 1985). This anti-resorptive effect was especially pronounced in studies using salmon CTN which has a 50-fold higher potency than mammalian CTN (Chambers and Moore 1983; Zaidi et al. 2002). Because of these findings, CTN was and still is considered as functional counterpart of PTH. Although pharmacologic actions were studied extensively and seem to be clearly described, there is marked uncertainty about calcitonin’s function in mammalian physiology (Davey and Findlay 2013; Hirsch et al. 2001). This is mainly based on the fact that patients with CTN deficiency following thyroidectomy do not display the expected osteoporosis and that bone mineral density was found decreased in individuals with medullary thyroid carcinoma (MTC) (Hurley et al. 1987; Zaidi et al. 2002).
Whereas there is an ongoing lively debate about its role as a regulator of calcium and bone metabolism, calcitonin’s diagnostic importance as a tumor marker for MTC is undoubted. It is well known that patients with MTC display increased basal and pentagastrin or calcium stimulated CTN concentration compared to patients with C-cell hyperplasia (CCH) or healthy subjects (see Chaps. 2 and 3 of this article). Before the era of RET mutation identification, serum mature calcitonin measurements were the only mean to identify affected relatives in the case of familiar MTC (Bihan et al. 2003).
Besides the mature monomeric CTN (1–32 amino acids), several other isoforms, precursors, and metabolites are detectable in the circulation (Bucht et al. 1985; Deftos et al. 1975; Schifter 1993; Tobler et al. 1983). The half live of these isoforms distinctly differ between 15 min for mature CTN and several days for some CTN precursors (Becker et al. 2002). However, most of CTN immunoreactivity is referred to the intact molecule. Otherwise, patients with MTC display a big heterogeneity of calcitonin immunoreactivity (Austin and Heath 1981; MacIntyre et al. 1984; Snider et al. 1977; Tobler et al. 1983). Compared to healthy subjects, polymeric forms as well as monomeric form of calcitonin can be found in patients with MTC. Polymeric forms of CTN in MTC patients may be a result of intermolecular disulfide bridges or disulfide bridges to unrelated proteins (Goltzman and Tischler 1978). Nevertheless, Austin and Heath (1981) could demonstrate that the polymeric isoforms are less bioactive than the monomeric one. In patients with chronic renal failure, monomeric CTN is relatively decreased, whereas increased concentrations of high molecular weight isoforms of CTN precursors and metabolites, probably generated through homo- and heteroaggregates, can be detected (Lee et al. 1977). Dialysis in patients with chronic renal failure contributes further to the heterogeneity of CTN, because high molecular weight forms are not dialyzable and accumulate due to the dialysis-associated calcium load. This should be considered when determining CTN concentration in patients with chronic renal failure. As mentioned before, measurements of CTN concentration are of considerable value in preoperative diagnosis of MTC (Bihan et al. 2003). Furthermore, preoperative mature CTN levels in MTC may also be indicative of tumoral volume and predictive of eventual cure when less than 50 pg/mL (Cohen et al. 2000). In addition, CTN is a marker for the postoperative prognosis of patients with MTC. In particular, monomeric CTN seems to be exclusively secreted by persisting tumor cells of the MTC (Engelbach et al. 1998). Hence, CTN concentrations of patients with total thyroidectomy measured with assays specific for the monomeric isoform suggest incomplete tumor extirpation (Engelbach et al. 1998). Thus, the heterogenic patchwork of CTN immunoreactivity should be clearly separated by the used assay system to obtain reliable values for diagnostic purpose (Bertagna et al. 1980; Heath and Sizemore 1979; Morimoto et al. 1981; Singer and Habener 1974; Sizemore et al. 1975; Voelkel and Tashjian 1971).
1.2 CTN Assay Methods
Measurement of CTN is described to be performed in general by immunoassay methods. The first radioimmunoassays (RIA) for CTN were established in the late sixties of the last century (Cooper et al. 1967; Deftos et al. 1968; Deftos 1971; Hirsch et al. 1964). These assays were competitive and exhibited frequently a low specificity. Later, two-sided immunoassays (immunoradiometric assays, IRMA) with a distinctly improved specificity became more and more accepted. These assays used two different antibodies directed against spatially separated, specific epitopes of the CTN molecule (Body and Heath 1984; Motté et al. 1988). Around the year 2000, assay manufacturers have moved from radiolabeled systems to fluorescent and chemiluminescent assays (Bieglmayer et al. 2002; d’Herbomez et al. 2001). Thus, the inconvenient radioactivity was substituted by labels with a somewhat higher signal efficiency to receive an improved analytical sensitivity. In the following years, the chemiluminescence test of the manufacturer Nichols Institute Diagnostics (NID) was considered as “gold standard” for CTN measurements as its functionality has been evaluated in a number of papers (Bieglmayer et al. 2007; Grauer et al. 1998; Engelbach et al. 2000). However, NID terminated the production of their immunoassays recently. Moreover, due to the augmenting automation of hormone analytics in laboratory diagnostics of the last years, the number of fully mechanized systems increased noticeably and their analytical quality as well as clinical validity has been proven in recent papers (Biegelmayer et al. 2007; Kratzsch et al. 2011). Nevertheless, there are still some IRMAs on the market with a sensitivity and specificity that is approximately comparable to fully mechanized systems.
In the following important key points in assay, methodologies for CTN measurement are discussed:
Calibration: The unique calibrator 89/620 presented by the World Health Organization (WHO) is available as the ultimate basis preparation. Most of the manufacturers but also some in-house assays used calibrators that have been adjusted on this tool as a first precondition for the comparability of results from CTN assays (Camacho et al. 2014; Kratzsch et al. 2011).
Analytical sensitivity is a further important point of interest, especially for the detection of remaining tissue in the follow-up after thyroidectomy from MTC patients: The value of sensitivity was reported for most of the recently available assay methods in the range between 0.5 and 2.5 pg/mL (Camacho et al. 2014; d’Herbomez et al. 2001; Kratzsch et al. 2011; Roche cobas 2015).
Precision: Coefficients of variation (CV%) were described below 5.9 % for CTN concentrations of fully mechanized systems in the normal or minimal increased range of concentrations (Bieglmayer et al. 2007; Kratzsch et al. 2011). The respective data of manually handled assays were <12.9 % and thus in general, slightly higher (Bieglmayer et al. 2002; Camacho et al. 2014; Saller et al. 2002).
Cutoff and reference range: In the past, there was a consensus that patients with a calcitonin concentration higher than 10 pg/mL should undergo a PG test to exclude MTC (Motté et al. 1988). Recently, manufacturers of commercially available CTN assays revised this cutoff or decision point for the diagnosis of hypercalcitoninemia to values below 21 pg/mL, what is identical with the 95th or 97.5th percentile of reference or normal subjects. These data were gender-dependent with distinctly elevated values in male volunteers due to their higher thyroid volume. For males, commercially available CTN methods presented a cutoff in a range between 21 pg/mL (IRMA, Scantibody Laboratories) and 8.4 pg/mL CTN (IL2000, Siemens). In contrast, respective data for females were between 10 pg/mL (IRMA, Medipan) and 5.5 pg/mL (Liaison, Diasorin). In the literature, even higher cutoffs of up to 32.8 pg/mL for males and 14.6 pg/mL for females have been described (Rink et al. 2009).
Comparability of CTN concentrations from different assay methods: The above-mentioned differences in cutoff and reference range can be attributed to differences in the functionality of the assay constituents. Despite the widely use of the calibrators adjusted on the unique CTN WHO reference preparation 89/620, antibodies of commercially assays appear to present their interaction with a series of epitopes from different regions of the CTN molecule. Moreover, various labels for the signaling antibody may partially hamper antigen–antibody interactions. Nevertheless, numbers of preanalytical, analytical, and postanalytical influencing and interfering factors may systematically modify assay results (see next chapter). The differences between samples measured by different assay methods can be estimated in external quality control (QC) surveys. The last German survey [Reference Institute for Bioanalytics (RfB), 4/2014] revealed CTN concentrations (mean ± standard deviation (SD)) for measurements of all participants (n = 170; 13 different methods) of 23.7 ± 3.38 pg/mL with a total CV of 14.3 % for sample A and of 35.3 ± 6.87 pg/mL with a total CV of 19.4 % for sample B. This data pretend an acceptable overall comparability of CTN data; however, a high intermethod variability between specific methods cannot be excluded. Thus, the mean of all measurements of the assay from the manufacturer with the highest CTN results of sample B was 42.5 pg/mL, whereas the assay with the lowest measuring values delivered a mean of only 24.3 pg/mL. A systematic bias was also shown for the comparison between assays with monoclonal and polyclonal antibodies (Tommasi and Raspandi 2007a, b). Moreover, results of CTN from different assay methods depend especially on the considered hormone concentration and on the source of sample used (Kratzsch et al. 2011).
1.3 Influencing Factors on CTN Measurement
The serum calcitonin concentration range of subjects without thyroid diseases is usually considered to be below 10 pg/mL (Costante et al. 2009). Calcitonin levels higher than cutoff may indicate preoperative MTC, postoperative recurrence of MTC, or untreated metastases (Hahm et al. 2001; Pacini et al. 1994). For some patients who have normal or slightly elevated basal calcitonin findings and clinically suspected MTC, calcitonin stimulation tests are performed, usually with PG or calcium (see Chaps. 2 and 3). Stimulated calcitonin values at PG test higher than 500 pg/mL are a clear indicator of MTC. However, intermediate calcitonin levels between 10 and 500 pg/mL cannot guarantee the presence of MTC due to various influencing factors.
Influencing factors on the determination of calcitonin concentration can result in false-positive diagnosis which might lead to unnecessary thyroidectomy or in false-negative diagnosis which can cause poor prognosis of MTC. Therefore, it is of significance to consider possible influencing factors in interpreting calcitonin results. In this chapter, the influencing factors are largely categorized into four sections: (1) analytical factors, (2) physiological factors, (3) pharmacological factors, and (4) pathological factors.
1.3.1 Analytical Factors
Sandwich immunoassays are applied when measuring calcitonin in the clinical laboratory. In immunoassays, falsely low results can be obtained due to unsuited storage conditions for the serum sample and the hook effect. Falsely elevated results can be obtained due to cross-reactivity with procalcitonin or interference by heterophilic antibodies.
CTN is one of the most susceptible biomarkers in laboratory routine analysis. At a room temperature of 20–25 °C, the CTN concentration remained constant only for a couple of hours. Accordingly, it is highly suggested to store any blood sample containing the CTN molecule at a temperature between 4 and 8 °C for up to 6 h or in the frozen state for long-term storage (Kratzsch et al. 2011). Interestingly, 4 freeze/thaw cycles did not influence the CTN result.
Hook effect can be detected if the CTN result of a diluted sample is higher than that of the undiluted sample. The reason for such a particular effect is an overload in the binding capacity of the solid-phase adsorbed antibody due to an extraordinary high concentration of the analyte that inhibits binding activity of the labeled second antibody. Leboeuf et al. (2006) reported that a calcitonin IRMA exhibited such a hook effect and added that a falsely too low calcitonin result could dramatically change the prognosis of the patient. Tommasi and Raspanti (2007a, b) evaluated 3874 CTN measurements using a simultaneous polyclonal CTN IRMA that delivered 131 clinical serum samples above cutoff and, therefrom, 22 samples with a nonlinear relationship at several dilution steps. A nonlinear dilution can be indicative for a hook effect or for a dissociation of CTN aggregates. Although such effects occur infrequently in sandwich immunoassays, in suspicion of falsely decreased results, serial dilution of samples with increased CTN values should be performed until a stable quantitative response is achieved.
Procalcitonin is a peptide precursor of calcitonin molecule. One of the limitations in immunoassays is the cross-reactivity with the compounds which have structural similarity to the target molecule. Kratzsch et al. (2011) reported that the frequency of increased calcitonin concentrations was related to the degree of cross-reactivity exhibited by an specific CTN IRMA with PCTN. PCTN increased in patients with general infections but may also be elevated in states with non-infectious inflammation. Uhrova et al. (2011) demonstrated that there was visible interference of PCTN in the CTN determination in septic patients (PCTN ˃ 0.5 ng/mL) with the IRMA method.
Interference by heterophilic antibodies in immunoassays is also a source of potential errors in calcitonin immunoassays. Heterophilic antibodies can link detection and signal antibody in sandwich immunoassays leading to enhanced immunoreactivity and consequently to increased measuring signals and analyte concentrations (Tate and Ward 2004). Papapetrou et al. (2006) suggested that the interference by heterophilic antibodies should be always considered when an unexpected increase in serum CTN concentrations is detected or when a patient has a high basal CTN and minimal CTN response to PG- or calcium stimulation tests. Interference by heterophilic antibodies can be also identified by nonlinearity in serial serum dilution results. In order to eliminate the interference by heterophilic antibodies, normal mouse gamma globulins or heterophilic blocking agents can be added.
1.3.2 Physiological Factors
Physiological factors which can influence the CTN concentrations can be viewed in light of age, sex, food intake, and lifestyle habit such as cigarette smoking or alcohol consumption.
CTN has been shown a progressive decrease with age: Concentrations were relatively high in neonates, declined from 6 months of age, and reached the adult levels almost at the age of 3 (Basuyau et al. 2004). Reference intervals for children were rarely published for currently available assay methods: Only one paper suggested <40 pg/mL for children under 6 months of age, <15 pg/mL in children between 6 months and 3 years of age, and over 3 years of age the values should be indistinguishable from those observed in adults (Basuyau et al. 2004).
In adults, CTN was found generally higher in men than in women. Reference intervals are dependent on the used assay method and were suggested to be gender-dependent (see Sect. 1.2). A postmortem study has shown that physiologically men have twice as many C-cells as women (Engelbach et al. 2000). Sex difference in calcitonin is more dramatic during PG stimulation test (see Chap. 3). Heath and Sizemore (1977) showed that women had significantly lower CTN levels and a lesser response to both calcium- and PG stimulation tests than men with a statistical significance.
Zayed et al. (2013) reported that serum CTN concentration was significantly increased by food intake in healthy young subjects and revealed a circadian rhythm with increased values during the afternoon. This study suggests that timing of blood sampling relative to meals should be considered in CTN measurements. Polymeris et al. (2011) have shown that hyperinsulinemia during oral glucose tolerance test and high normal serum cortisol were also associated with increased secretion of CTN in normal subjects within the physiological range. Therefore, physicians should be aware that in states of acute hyperinsulinemia or high but even still within normal range measured serum cortisol, CTN results may be higher than expected without the presence of MTC or CCH.
d’Herbomez et al. (2007) reported that CTN levels were higher in smokers than in non-smokers. C-cells are distributed not only in the thyroid and the thymus but also in the liver, lungs, duodenum, and jejunum. It has been demonstrated that tobacco increased the number of neuroendocrine cells and the secretion of peptides such as CTN (Kapoor and Jones 2005). Alcohol was also shown to increase CTN (Kanis et al. 1979). Vantyghem et al. (2007) observed an increase in CTN concentrations in patients with chronic alcohol and smoking. Interestingly, the hormone concentration did not normalize after the withdrawal of alcohol. From this finding, it was suggested that smoking may play more predominant role than alcohol consumption in CTN elevation (Vantyghem et al. 2007).
1.3.3 Pharmacological Factors
Prolonged treatment with histamine-2 receptor blockers (H2RB) and/or proton-pump inhibitors (PPI), glucocorticoids, and several drugs have been associated with hypercalcitoninemia. CTN medication used for a therapeutic purpose should also be checked for the interpretation of CTN serum concentration.
H2RB/PPI drugs decrease gastric acid secretion in patients with hypergastrinemia, pernicious anemia, selective gastric vagotomies, and gastric operations. Inhibition of gastric acid elevates endogenous gastrin levels. Gastrin is secreted from G cells and known to stimulate CTN levels. PG used in the CTN stimulation test is a synthetic gastrin analog. Erdogan et al. (2006) found significantly higher stimulated CTN levels in patients with H2RB and PPI therapy than controls. Chronic use of omeprazole has been reported to raise CTN levels after 2–4 months of therapy (Freston 1994; Klinkenberg-Knol et al. 1994).
Acute use of glucocorticoids has been shown to increase basal and calcium-stimulated CTN in patients with arteritis after 1 week of treatment with prednisolone (Mulder et al. 1990). However, CTN secretion capacity significantly decreased after 6 weeks of prednisolone therapy compared to the initial level. Exhaustion of CTN response system during the chronic use of glucocorticoids later contributes to the glucocorticoid-induced osteoporosis. For the treatment of glucocorticoid-induced osteoporosis, CTN can be used for a therapeutic purpose.
Other than these, ß-blocker, glucagon, enteroglucagon, and pancreozimine were reported to raise serum CTN levels (Toledo et al. 2009).
1.3.4 Pathological Factors
There are several physiological and pathological conditions in which CTN concentrations increase without the presence of MTC or CCH. Hypercalcitoninemia has been frequently reported in chronic kidney disease (CKD), neuroendocrine tumors, hypergastrinemia, hypercalcemia, and chronic autoimmune thyroiditis.
Among patients with CKD, CTN concentration was elevated in about 30 % of patients (Escalada et al. 1993; Garancini et al. 1983; Kotzmann et al. 1999; Kratzsch et al. 2011; Mulder et al. 1982; Niccoli et al. 1995). The amount of CTN in the circulation may increase due to the lowered clearance rate of CTN (Niccoli et al. 1995). Borchhardt et al. (2005) demonstrated that PG-stimulated CTN of around 100 pg/mL normalized after successful kidney transplantation. Martínez et al. (1983) reported that patients with CKD showed significantly lower calcium and CTN concentrations after receiving continuous ambulatory peritoneal dialysis.
Since C-cells are distributed throughout the neuroendocrine tissues, several types of neuroendocrine tumors of the lung or gastrointestinal tract have shown an elevated concentration of CTN (Becker et al. 2004). However, chronic hypercalcemic state can exhaust CTN reserves and diminish the CTN response to calcium stimulation test. Kim et al. (2014) reported such a case of prostatic and multiple bone metastases from medullary thyroid cancer, in which serum CTN levels were not significantly increased in response to an acute intravenous calcium injection.
Since gastrin is a strong stimulant of synthesis and stimulation of CTN, patients with hypergastrinemia may exhibit high concentrations of serum CTN (Toledo et al. 2009). In the same way, hypercalcemia caused by primary hyperparathyroidism, malignancy, vitamin-D metabolic disorders, or renal failure has been associated with hypercalcitoninemia (Toledo et al. 2009).
Chronic autoimmune thyroiditis has been reported leading to increased CTN. However, the findings were in part inconsistent. Uwaifo et al. (2001) reported that a patient with Hashimoto’s thyroiditis showed an elevated calcitonin level with no evidence of MTC. Barbot et al. (1991) reported that 3 out of 24 patients with Hashimoto’s thyroiditis showed high calcitonin levels. From pathological examination after thyroidectomy, high calcitonin levels were found to be a consequence of extensive C-cell hyperplasia in Hashimoto’s thyroiditis. Guesgen et al. (2013) reported there is no correlation between Hashimoto’s thyroiditis and high calcitonin levels. Elevation of CTN in 1–3 % of patients with the same diagnosis has been observed by Kratzsch et al. (2011).
1.4 PCTN Assays
There is almost only one PCTN measurement principle commercially available that had been developed by the manufacturer “Brahms” in the year 2000 and which is sold by “Thermo Scientific” recently. Moreover, the two independent manufacturers, “USDN” and “Biomerioux”, can deliver manually performed kits of this parameter (Chan and Gu 2011). An analytical sensitivity of 60 pg/mL as presented by the fully mechanized system, Kryptor (Thermo Scientific), is highly sufficient for the use of PCTN as biomarker for MTC diagnosis. To avoid purchasing a complete clinical analyzer only for PCTN measurement, the manually performed assays can be used alternatively with somewhat inferior quality control data (Steinbach et al. 2004). Reference cutoffs were set at 250 pg/mL or 500 pg/mL to indicate a potential bacterial infection or general inflammation, and levels higher than 500 pg/mL point a high probability of a bacterial infection. PCTN concentrations of non-MTC and of MTC patients correlated with CTN as calculated by “r” values between 0.36 and 1.0 in dependence on the concentration range of CTN values that were included into the calculation. The whole spectrum of data points delivered excellent correlation data (Kratzsch et al. 2011). Intra- and Interassay precision was found to be lower than 5 and 10 % for the mechanized as well as 7 and 17 % for the manually performed methods (Steinbach et al. 2004). The main advantage of using PCTN compared to CTN is the distinctly higher stability of the precursor molecule at room temperature and at 4 °C (Kratzsch et al. 2011; Steinbach et al. 2004). Thus, PCTN and CTN appear to have a comparable diagnostic capability to detect MTCs. However, “positive” PCTN values of more than 50 pg/mL may also be reached in subclinical infections and will lead, therefore, to an overdiagnosis of the tumor.
2 Screening for Medullary Thyroid Carcinoma in Patients with Struma Nodosa
2.1 CTN Screening
There is a lot of evidence that the measurement of serum CTN concentrations in patients with thyroid nodules can lead to an earlier diagnosis of MTC or CCH compared to the exclusive use of imaging procedures and fine-needle aspiration cytology (FNAC). In the following, some selected studies will be cited and issues about the diagnostic use of CTN as a screening tool will be discussed.
In one of the biggest studies, 10,864 patients with nodular disease were included during the years 1991–1998 in Italia (Elisei et al. 2004). Using CTN screening, the prevalence of MTC was determined with 0.4 %. The observed positive CTN concentrations had higher diagnostic sensitivity than FNAC and allowed the diagnosis of MTC at an earlier stage compared to a group of MTC patients diagnosed only with FNAC and by postsurgical histological examination. Biochemical cure and tumor remission in the follow-up were clearly better in the group with CTN screening.
These data were supported by another large study with a cohort of 5817 consecutive patients demonstrating thyroid nodules (Costante et al. 2007). The authors estimated the positive predictive value (PPV) of CTN values in the preoperative diagnosis of MTC via gradual limits for this parameter: 100 % for CTN >100 pg/mL; 25 % for CTN between 50 and 100 pg/mL; 8.3 % for CTN between 20 and 50 pg/mL; and 23.1 % for CTN >20 pg/mL. The relatively low PPV values in the range between 20 and 100 pg/mL, due to the low incidence of MTC, caught up a confirmatory test like PG stimulation (PPV 40 % for CTN >100 pg/mL). The importance of this suggestion was supported by data of Hahm et al. (2001). From 1448 patients with nodular goiter screened for CTN, 56 revealed to have a basal elevation, but 46 had no evidence for a MTC. This deficit in the diagnostic value of CTN screening could be overcome by the confirmation test. The PG test, done in this study, demonstrated all of the 10 MTC patients with a CTN concentration higher than 100 pg/mL, whereas FNAC suggested MTC in only 2/9 patients. Contrasting results were delivered in a recent study that included 2773 patients before surgical treatment of nodular thyroid disorders and suspected MTC (Chambon et al. 2011). CTN screening was performed and PG stimulation was carried out if CTN was >10 pg/mL. Interestingly, the latter test delivered no further diagnostic information additional to the CTN screening value. MTC was observed in 12 patients, always present when basal CTN was >60 pg/mL (n = 8). In the range between 10 and 60 pg/mL, 4 patients had the diagnosis MTC. Two additional cases with micro-MTC were detected by histopathology only, and CTN was below 10 pg/mL in both cases. Comparable results were described for the investigation of 5920 thyroidectomies: A group of 14 patients with nodular thyroid disease and CTN concentrations between 20 and 100 pg/mL was identified (Boschin et al. 2014). MTC (n = 9) was diagnosed by FNAC and histopathology, whereas CCH revealed to be the finding in the remaining five. A PG test with CTN >100 pg/mL confirmed MTC in 5/9 cases but pointed to a questionable diagnosis MTC in 2/4 cases with HCC. Accordingly, a clear separation of these two diagnoses appeared to be impossible by PG test. Taken together all these and some other data [see reviews from Daniels (2011) and from Ahmed and Ball (2011)]: CTN screening in patients with nodular goiter delivers increased values for both MTC and HCC. Basal CTN concentrations higher than 60–100 pg/mL are highly indicative for the diagnosis MTC. In the range between cutoff and 60 pg/mL CTN, both MTC and HCC may be a relevant diagnosis. FNAC and stimulation test may but not must be preoperatively helpful tool for the further characterization of patient’s disease. Accordingly, analysis of CTN is advisable in all patients with documented thyroid nodules according to the German evidence-based consensus recommendation (Karges et al. 2004) and the European Thyroid Cancer task force (Pacini et al. 2006), whereas the European Thyroid Association weakened this recommendation to “nonstimulated CTN level may be considered before thyroid surgery for nodular goiter” (Gharib et al. 2010). As the low PPV of slightly elevated CTN values is still under debate, the American Thyroid Association Guidelines does neither recommend nor dis-advise this procedure (Cooper et al. 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree