The Elderly Heart
Ageing of the heart is associated with the deposition of amyloid in the atrial myocardium, a gradual loss of the specialized pacemaker myocytes of the sinoatrial (SA) node and the deposition of collagen and fibrous tissue in the specialized ventricular conduction tissue. These ageing processes are associated with a pathological outcome in some patients. For instance, loss of SA node pacemaker cells contributes to the development of sick sinus syndrome. This process is augmented by pathological changes secondary to cardiovascular disease such as hypertension and coronary atherosclerosis, both of which increase in incidence with age. In reviewing 12-lead and 24 h electrocardiogram (ECG) survey findings in the elderly, it can be difficult to distinguish between whether purely ageing or pathological processes are responsible.
12-Lead and Ambulatory ECG Surveys
Findings of a prolonged PR interval, left-axis deviation and bundle branch block (BBB) are more common in the elderly. First-degree heart block, as a lone finding in an asymptomatic patient, is not associated with an adverse prognosis. Left-axis deviation is associated with cardiovascular disease but in the absence of clinical disease does not carry a worse prognosis. Increases in left ventricular hypertrophy and left BBB with age correspond to the increased incidence of cardiovascular disease and clearly are associated with an increased mortality. Right BBB is more common than left in the elderly, but has no prognostic impact per se.
In the absence of medication, a sustained bradycardia is not a normal finding in the elderly. However, elderly subjects do show a significant reduction in 24 h heart rate variability.1 A survey of 500 asymptomatic individuals aged 50–80 years2 found no association between heart rate and age, and a study of 1372 individuals aged 65 years and older3 found no association between bradycardia and age. Various studies have shown a small decline in mean heart rate with age,4 but even in healthy subjects aged 80–99 years, mean heart rate is still >70 bpm.1 A persistent and significant bradycardia should alert the clinician to the possibility of sinus node disease. There is a low incidence of sinus arrhythmia in the elderly, but ectopic activity is considerably increased. Specific brady- and tachyarrhythmias are discussed individually below.
Symptomatic Bradycardias
Cardiac impulse originates in the SA node, propagates through the atria and is normally conducted to the ventricles via the atrioventricular (AV) node and bundle of His. Bradycardia can result from abnormalities at any level: dysfunction of SA node automaticity, conduction disturbances within the AV node or bundle of His. These are discussed individually below. Bundle branch or fascicular blocks may prolong ventricular depolarization (QRS complex width) or cause electrical axis deviation, but will not result in a bradycardia unless conduction through all fascicles is interrupted, equivalent to complete AV block. The autonomic nervous system regulates sinus node automaticity and AV nodal conduction. The balance of parasympathetic and sympathetic tone is subject to influence from a host of extrinsic factors: physiological [e.g. sleep (see Chapter 54, Sleep apnoea and sleep disorders)], pathological [e.g. hypothyroidism (see Chapter 98, Thyroid disorders)] and iatrogenic (e.g. medication). The causes of a bradycardia are summarized in Table 36.1.
Table 36.1 Causes of bradycardia.
| System | Cause |
| Cardiovascular | Ischaemia/infarction |
| Infiltrative disorders (e.g. sarcoid, amyloid, haemochromatosis) | |
| Inflammatory disorders (e.g. systemic lupus erythaematosus, rheumatoid) | |
| Respiratory | Obstructive sleep apnoea |
| Medication | β-Blockers (including topical eye preparations) |
| Anti-arrhythmics (e.g. digoxin, amiodarone) | |
| Antihypertensives (e.g. calcium channel antagonists) | |
| Iatrogenic | Valve replacement |
| Catheter/surgical ablation | |
| Correction of congenital heart disease | |
| Endocrine | Hypokalaemia |
| Hyperkalaemia | |
| Hypothyroidism | |
| Neurological | Raised intracranial pressure |
| Increased vagal tone (e.g. micturition, defecation, coughing) | |
| Carotid sinus hypersensitivity | |
| Infectious disease | Infective endocarditis |
| Lyme disease | |
| Chagas disease | |
| Miscellaneous | Hypothermia |
| Metastatic disease |
SLE, systemic lupus erythaematosus.
Presentation
Episodes of bradycardia are common in all age groups. However, a sustained bradycardia is not a normal finding in the elderly and, as discussed above, implies pathology. The presence or absence of symptoms is principally determined by the heart’s ability to compensate for a bradycardia. Cardiac output is the product of heart rate and left ventricular stroke volume. If the latter cannot increase sufficiently to match demand and peripheral and/or cerebral perfusion falls, this will result in symptoms. These vary from understated symptoms such as lack of concentration, fatigue, poor memory, dizziness and myalgia to more pressing concerns of syncope and heart failure.
Assessment
Documentation of a bradycardia (rhythm strip, 12-lead ECG or ambulatory monitoring) is not sufficient. The symptoms described above are all non-specific and differential diagnoses are extensive, especially in the elderly. It is essential to establish a correlation between the patient’s symptoms and the occurrence of bradycardia, otherwise treatment of the arrhythmia may be successful (e.g. pacemaker implantation), but without any symptomatic benefit. History, examination and investigations should be targeted at confirming the presence of arrhythmia, its association with symptoms, to differentiate physiology from pathology and to recognize reversible risk factors (such as medication, hypothyroidism, sleep apnoea).
Sinus Node Dysfunction (Sick Sinus Syndrome)
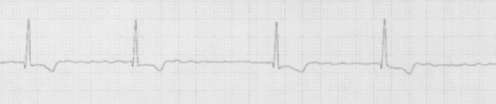
The term sick sinus syndrome confers the impression of a constellation of multiple electrocardiographic manifestations such as sinus bradycardia, inappropriate sinus node response to exercise (chronotropic incompetence), sino-atrial block and periods of sinus arrest, particularly occurring after paroxysms of atrial tachyarrhythmias (‘tachy-brady syndrome’). Dysfunction may progress to the stage that no sinus beats occur (Figure 36.1). Both paroxysmal and chronic atrial fibrillation are commonly associated with sinus node disease. Sinus node dysfunction is primarily a disease of the elderly resulting from degenerative or ischaemic causes which may also affect other conductive tissue including the AV node. The majority of patients experience recurrent syncope from sinus pauses with inadequate escape rhythm or marked sinus bradycardia. Mortality appears to be unaffected by sinus node dysfunction5 and survival is influenced by associated pathology such as ischaemic heart disease.
Figure 36.1 Junctional bradycardia: failure of sinus beats has resulted in a junctional escape rhythm. The junctional impulses are seen conducting antegradely causing ventricular depolarization, but also retrogradely into the atria causing inverted P waves, seen immediately after the QRS complex (arrows).
The main indications for pacing in sinus node dysfunction are as follows:6
- Sinus node dysfunction with documented symptomatic bradycardia, symptomatic chronotropic incompetence or where bradycardia is the result of medication necessary to treat other medical conditions, such as in the treatment of tachyarrhythmias (class I recommendation).
- Sinus node dysfunction and heart rate <40 bpm, where an association between symptoms consistent with bradycardia and the presence of bradycardia has not been clearly established; syncope of unknown origin in presence of significant sinus node abnormalities on electrophysiological testing (class IIa recommendations).
Atrioventricular Blocks
First-Degree Heart Block
The PR interval (time from onset of P wave to onset of QRS complex) corresponds to the time from initiation of atrial depolarization, conduction through the atria and into the bundle branch system via the AV node and bundle of His. A prolonged PR interval (>0.2 s) with preservation of 1:1 AV conduction is termed first-degree heart block. The incidence of first-degree AV block increases with ageing. Moderate prolongation of the PR interval in this fashion is a benign condition,7 but PR intervals >0.3 s can be symptomatic.8 Marked first-degree AV block could cause loss of synchrony between the atria and the ventricles leading to incomplete atrial and ventricular filling and increased capillary wedge pressure.
Indications for pacing in first-degree AV block are as follows:6
- First-degree heart block associated with symptoms of a delay in AV synchronous contraction or haemodynamic compromise (class IIa recommendation).
- First-degree heart block in association with neuromuscular diseases due to unpredictable progression of AV disease; drug-related AV block where the block is expected to persist even after withdrawal of the drug (class IIb recommendation).
Second-Degree Heart Block
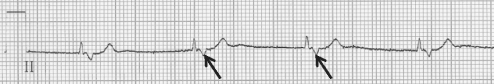
This is an intermittent failure of atrial depolarization to result in ventricular depolarization and tends to occur in various patterns. Mobitz type I second-degree heart block (Wenckebach) is present when there is progressive lengthening of the PR interval with each beat until an atrial depolarization is not conducted, resulting in a dropped beat. The PR interval resets and the cycle resumes. Mobitz type II second-degree heart block occurs when atrial depolarizations are intermittently blocked without preceding progressive PR interval prolongation. AV conduction occurring in a 2:1 fashion (or higher) represents another pattern of second-degree heart block. If block of two or more consecutive P waves occurs, this is termed advanced second-degree heart block (see Figure 36.2).
Figure 36.2 Rhythm strip showing advanced second-degree heart block. The second ventricular complex is a junctional escape beat.
Indications for pacing in second-degree AV block are as follows:6
- Any form of second-degree heart block associated with symptomatic bradycardia or ventricular arrhythmias presumed to be secondary to the AV block (class I recommendation).
- Advanced second-degree AV block in symptom-free patients with documented significant periods of pauses or asystole lasting ≥3.0 s or any escape rhythm of rate <40 bpm in an awake patient (class I recommendation).
- Advanced second-degree AV block associated with arrhythmias and other medical conditions that require drug therapy that results in symptomatic bradycardia (class I recommendation).
- Unresolving advanced second-degree block following cardiac surgery or catheter ablation of AV junction (class I recommendation).
- AV block during exercise in the absence of myocardial ischaemia.
- Type II second-degree heart block with a wide QRS complex (class I recommendation).
- Asymptomatic type II second-degree heart block with narrow QRS complex (class IIa recommendation).
- Associated with symptoms of a delay in AV synchronous contraction or haemodynamic compromise (class IIa recommendation).
- In association with neuromuscular diseases due to unpredictable progression of AV disease; drug-related AV block where the block is expected to persist even after withdrawal of the drug (class IIb recommendation).
Third-Degree Heart Block (Complete Heart Block)
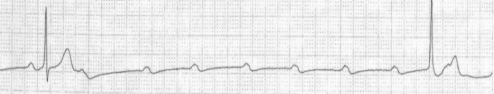
Third-degree heart block is a complete block of conduction between the atria and ventricles resulting in regular atrial activity and the presence of an independent escape rhythm. The escape rhythm is generally ventricular in origin with a wide QRS complex and rate of ∼30–40 bpm. Nodal or junctional escape rhythms imply that the anatomical level of block is higher within the AV node or the bundle of His (Figure 36.3). The lower the origin of the escape rhythm, the less specialized and hence less reliable is the conduction tissue.
Figure 36.3 Complete heart block. Rhythm strip showing P waves (arrows) that are independent and unrelated to QRS complexes. The QRS complexes are relatively narrow, suggestive of junctional escape rhythm.
Indications for pacing in complete AV block are as follows:6
- Third-degree heart block with one of the following features: symptomatic bradycardia; documented asystole ≥3.0 s or any escape rhythm of rate <40 bpm in symptom-free awake patients; unresolving block following cardiac surgery or catheter ablation of AV junction; asymptomatic third-degree heart block, especially in the context of LV dysfunction or cardiomegaly; AV block during exercise in the absence of myocardial ischaemia (class I recommendations).
- Asymptomatic heart block in the absence of cardiomegaly (class IIa recommendation).
- Drug-related AV block where the block is expected to persist even after withdrawal of the drug (class IIb recommendation).
Choice of Pacemaker
Once the decision to implant a pacemaker has been made, consideration should be given to the appropriate pacemaker mode. The choice lies between single-chamber ventricular-only pacing (VVI/R), dual chamber atrial plus ventricular pacing (DDD/R) or single-chamber atrial-only pacing (AAI/R). DDD or AAI pacing allow the preservation of normal physiology by maintaining AV synchrony. Single-chamber AAI pacing is indicated in patients with pure sinus node disease with no evidence of either existing or future development of disease elsewhere in the conduction system. However, elderly patients, who may initially present with apparently pure sinus node dysfunction, have a greater likelihood of more widespread conduction system involvement and therefore generally will not benefit from atrial-only pacing. Physiological pacing may improve haemodynamics, but dual-chamber systems can be technically more challenging as two leads are required with greater potential for late complications. A series of large prospective, randomized trials have compared ventricular pacing (VVI/R) with physiological systems (DDD/R or AAI/R) for sinus node dysfunction or AV block (see Table 36.2 for a summary of these trials). A key limitation of these trials is that a significant number of patients either crossed over between treatment arms or dropped out of their assigned pacing mode. Meta-analysis of these five trials shows that there is no difference in overall mortality between ventricular and physiological pacing systems and no difference in new-onset heart failure or improvement or progression of any existing heart failure.9
Table 36.2 Characteristics of trials comparing ventricular and physiological pacing.
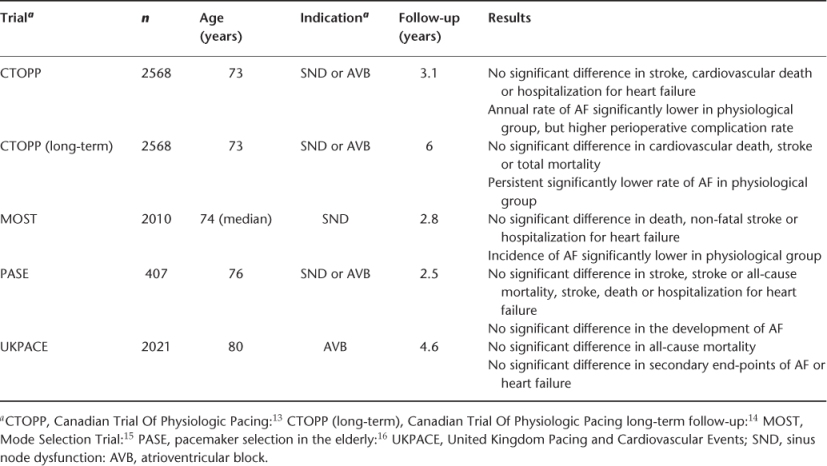
However, a statistically significant reduction in the development of AF was found with physiological pacing, particularly in the Mode Selection Trial (MOST) and Canadian Trial Of Physiological Pacing (CTOPP) trial. There was borderline reduction in thromboembolic stroke with physiological pacing. Some cross-over studies with intra-patient comparison between the two pacing modes have shown improved functional capacity and increased patient preference for dual-chamber pacing.10
Therefore, elderly patients with sinus node dysfunction who require pacing should be considered for a dual chamber system. No clear evidence exists for benefit of dual-chamber pacing over simple ventricular pacing for AV block in the elderly, but given the overall findings from trials, physiological pacing should be considered for those likely to be pacemaker dependent. Active elderly patients over 70 years of age appear to benefit from DDD pacing in terms of improvement in quality of life.11 However, the final decision in the elderly depends on an individual basis after taking into account patient preferences, co-morbidities and the available resources.
Complications of Pacemaker Implantation
Complications can occur during implantation and include pneumothorax (1–2% with subclavian vein approach; <0.1% with cephalic or axillary vein approach), bleeding, myocardial perforation and tamponade (<0.2%), lead dislodgement and failure to sense or capture. Post-procedure complications are bruising, wound haematoma, infection (<1%), device erosion, lead fracture and box or lead dislodgement. In a recent series, early complications (within 2 weeks of implantation) occurred in 6.7% of patients and late complications in 7.2% of patients.12 The majority of these patients required an invasive correction of the complication. Despite the reduction in size of the modern pacemaker, special considerations have to be made for elderly patients. Devices are generally implanted subcutaneously between the skin and the pectoral muscle in the infra-clavicular region, but skeletal deformities resulting from osteoarthritis or osteoporosis of the shoulder, spine or pectoral region may occasionally prevent this. In addition, wound healing and device erosion through the skin are more likely to occur with cachexia and thinning skin. In such patients, the pacemaker may be better positioned under the pectoral muscle. Clearly, as with any procedure, a risk–benefit assessment has to be made on an individual basis.
Atrial Tachyarrhythmias
Atrial Ectopic Beats
These are a common finding in the elderly and, if frequent, can result in a pulse that could be confused with AF. No specific treatment is required, but if symptomatic, patients generally respond to β-blocker therapy.
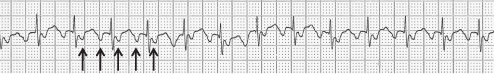
Atrial Tachycardia
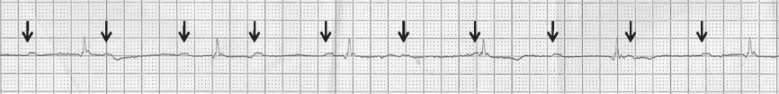
Short bursts of atrial tachycardia are common in the elderly (Figure 36.4). In many cases, no symptoms are associated and no treatment may be needed. Sometimes the atrial tachycardia triggers AF or causes rapid ventricular rates when treatment of the tachycardia may become necessary. Most elderly patients will achieve symptomatic benefit from β-blocker therapy, and some may require anti-arrhythmic medication (e.g. amiodarone, sotalol) or urgent direct current cardioversion (DCC) if haemodynamically unstable. Percutaneous catheter ablation utilizing radiofrequency or cryo energy is increasingly used, but success rates are generally higher in younger patients and therefore not currently a preferred option in the elderly.
Figure 36.4 Atrial tachycardia. Abnormal, inverted P waves are seen (arrows) with 2:1 conduction to the ventricles. The tracing may appear like atrial flutter, but note that the P wave rate or cycle length is less than the typical flutter cycle length of 300 bpm.
Multifocal Atrial Tachycardia (MAT)
Another common atrial arrhythmia in the elderly, multifocal atrial tachycardia (MAT), is characterized by the appearance of diverse P wave morphologies as complexes originate from different foci within the atria. This can result in irregular R–R intervals and hence clinically mimic AF. There is an association with chronic airways disease and drug toxicity (digoxin, theophyllines and tricyclic antidepressants). Treatment should be aimed at the underlying cause.
Atrial Flutter
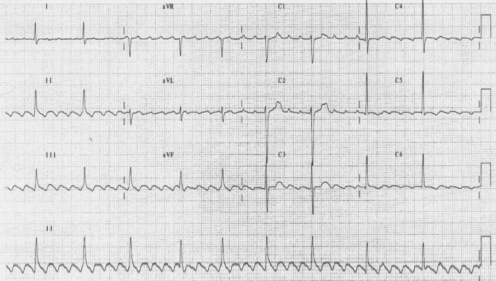
Atrial flutter and AF are two ends of the same spectrum. Whereas the atria are activated in a chaotic manner in AF, atrial flutter consists of organized atrial activation seen on the ECG as a regular saw-tooth pattern of flutter waves with typically a flutter-wave rate of ∼300 bpm (see Figure 36.5). A physiological 2:1 AV block frequently occurs and atrial flutter should always be suspected when a patients with a ventricular rate of 150 bpm. In the elderly, variable 4:1, 8:1 or other AV ratios may also be seen. Vagal manoeuvres or intravenous adenosine can temporarily increase the AV block, making flutter waves more visible. With typical (counterclockwise) atrial flutter, flutter waves are seen inverted in the inferior limb leads, giving rise to the characteristic saw-tooth appearance on the ECG. Atrial flutter commonly occurs in patients with AF and vice versa. Anti-arrhythmic medication prescribed for AF can convert the AF into atrial flutter. Management of the two conditions is essentially similar and although the stroke risk associated with atrial flutter may not be as high as for AF, there is still a substantial risk17 and a high likelihood of coexisting AF requiring the use of anticoagulation. In a given elderly patient, unlike other atrial arrhythmias, catheter ablation of typical atrial flutter may be performed with relative ease and high curative rates.
Figure 36.5 12-Lead ECG of typical atrial flutter with variable AV block. Note the inverted P waves (flutter waves) in the inferior leads II, III and aVF. The flutter wave cycle length is 300 bpm.
Atrial Fibrillation
AF is characterized by disorganized atrial activity confirmed on the ECG by the substitution of regular P-wave activity by rapid fibrillatory waves varying in shape, amplitude and timing. If AV node conduction is intact, the chaotic atrial activation will result in a rapid and irregular ventricular response. In the elderly, AV conduction may be impaired. A slower ventricular rate is common and sometimes verges on a symptomatic bradycardia (see Figure 36.6). The presence of regular rhythm on the ECG with a fibrillatory baseline implies development of complete AV block in a patient with AF, the regularity arising from the escape rhythm (Figure 36.7).
Figure 36.7 Lead II rhythm strip of a patient with chronic AF who presented with syncope. The rhythm is regular with fibrillatory baseline and absence of P waves, suggestive of complete heart block in the setting of AF.

Classification
AF can be classified into five types based on the presentation, duration and choice of treatment strategy. When the first episode is detected, it is important to understand that its duration may be uncertain and there may have been previous episodes, which were not symptomatic, remembered or documented. After first detection, AF is then subclassified into the following categories according to its time course and intervention: paroxysmal (PAF), persistent, long-standing persistent and permanent atrial fibrillation. PAF is characterized by recurrent episodes of AF alternating with sinus rhythm. The characteristic feature of PAF is that the episodes terminate spontaneously, usually within 48 h. If the episodes of fibrillation continue for more than 7 days or patients require intervention to restore sinus rhythm, this is termed persistent AF. When AF has lasted for ≥1 year and when it is decided to adopt a rhythm-control strategy, AF is termed long-standing persistent AF. Permanent AF is more a statement of intent rather than a duration or pathological based description and is reserved for patients in whom AF is resistant to conversion or accepted for rate control by the patient and there are no further attempts to achieve sinus rhythm. Permanent AF is redesignated long-standing persistent AF if there is a change of plan towards adopting a rhythm-control strategy.18
Epidemiology
AF affects 1–2% of the general population and is the commonest sustained arrhythmia in the elderly. The true prevalence of AF may be closer to 2% as many patients remain asymptomatic and may never present to a hospital. The prevalence of AF increases with age from <0.5% at 40–50 years to 5–15% at 80 years.19
Men are more often affected than women. An analysis of 1.4 million patients registered with 211 general practices in England and Wales showed that prevalence rates increased with age from <1 in 1000 in those under 35 years of age to >100 in 1000 in those aged 85 years and older.20 The prevalence of AF is estimated to double in the next 50 years.21
We are in part a victim of our own success owing to prognostic improvements made in coronary heart disease and heart failure, conditions known to predispose to the development of AF. This, together with an ageing population, has led to the description of a near-epidemic of AF.22 AF increases the risk of stroke fivefold. In 2000, the projected direct cost of AF to the UK National Health Service (NHS) was calculated at  459 million, 0.98% of total NHS expenditure,23 a conservative estimate as costs related to stroke rehabilitation and anticoagulant-related haemorrhage were not considered.
459 million, 0.98% of total NHS expenditure,23 a conservative estimate as costs related to stroke rehabilitation and anticoagulant-related haemorrhage were not considered.
Aetiology
Valvular AF
It is essential to make a distinction between valvular and non-valvular AF because of the consequence for stroke risk. Valvular heart disease is present in about 30% of patients with AF.24
Mitral stenosis and/or regurgitation, and in its later stages aortic stenosis, cause left atrial dilatation leading to AF. Rheumatic heart disease is now relatively rare in developed nations. In the Framingham Study, patients with rheumatic heart disease and AF had a 17-fold increase in stroke risk compared with age-matched controls.25
Non-valvular AF
AF occurring in the absence of rheumatic mitral stenosis or a prosthetic heart valve is termed non-valvular AF,18 which can then be further subdivided as follows.
With Associated Cardiovascular Disease
Hypertension (see Chapter 40, Hypertension), diabetes requiring medical treatment, heart failure (see Chapter 41, Heart failure), cardiomyopathies, coronary artery disease (Chapter 37, Ischaemic heart disease) and congenital heart defects such as atrial septal defect are commonly associated with AF. Of these, heart failure carries the highest predictive risk for the development of AF.26 Hypertension is identified as a risk factor for AF and related complications such stroke and thromboembolism.
Hypertension, when associated with left ventricular hypertrophy by electrical criteria on the ECG, strengthens its contribution towards predicting development of AF. Coronary artery disease can be both a reversible risk factor (ongoing ischaemia or infarction) or irreversible (scar formation from prior infarction).
Other Causes
Other identifiable and potentially reversible causes include obesity and obstructive sleep apnoea, electrolyte disturbance, sepsis, stress, hyperthyroidism, pulmonary disease, hypoxia and alcohol binge drinking. These factors need to be considered both in a first detected episode of AF and for the patient with a recent compromise in rate control.
Lone AF
Patients with a structurally normal heart in whom no identifiable cause can be found for their AF are denoted as having lone AF. This is probably rare in the elderly since almost all patients will have a degree of underlying heart disease by this stage and such a diagnosis of exclusion should only be made with caution in the elderly. In a small number of younger patients, sympathetic or vagal overstimulation may trigger AF and could influence the choice of anti-arrhythmic medication.
Consequences of AF
AF independently increases the mortality by twofold and is associated with stroke, other systemic thromboembolic disease, heart failure and related morbidity, leading to poor quality of life. Only antithrombotic treatment has been shown to reduce AF-related mortality.27
Atrial Remodelling
It is a common finding for patients presenting with PAF to cardiovert spontaneously to sinus rhythm within 24 h of its onset.
The success of electrical or chemical cardioversion and the subsequent maintenance of sinus rhythm are generally higher with AF of a shorter duration. These observations are consistent with the concept that AF itself is capable of inducing an electrical remodelling of the atria, which in turn sustains the arrhythmia. Electrophysiological artificial maintenance of AF in animal models has been shown to induce reversible atrial changes (shortened atrial refractoriness) that lead to the perpetuation of AF28 and eventually to histological (cellular dedifferentiation, fibrosis) and gross structural changes (atrial dilatation).
Haemodynamic Function
With the chaotic activation inherent in AF, synchronous atrial mechanical function is not possible. The left ventricle in AF can only fill passively in the absence the late diastolic contribution from atrial contraction. This in turn can lead to a 5–15% decrease in cardiac output, an effect that is more pronounced in patients with reduced ventricular compliance (e.g. hypertensives) in whom ventricular filling is significantly reliant on the atrial contribution. Further deterioration in haemodynamic function results from high ventricular rates due to shortening of the diastolic filling time and additionally tachycardia-related myopathy (tachycardiomyopathy) at rates persistently above 120–130 bpm. Restoration of atrial mechanical function after successful ventricular rate control or cardioversion leads to quantifiable improvements in left ventricular function.29 However, the return of atrial contraction may be delayed or insufficient if AF has been present for a substantial period of time.
Thromboembolism
The risk of stroke in non-rheumatic AF patients is 5.6 times greater than in age matched controls with an identical blood pressure distribution.25 Thrombus formation, often in the left atrial appendage (LAA), is responsible for embolic stroke and systemic embolism in the context of AF. Stroke risk consistently and significantly increases with age from 6.7% in those aged 50–59 years to 36.2% for those aged 80–89 years.30 Asymptomatic cerebral infarction based on computed tomography (CT) findings has been found in 14.7–48% of AF patients.31–33 The large variation in incidence is probably due to the use of different radiological definitions of infarction and study size. The two largest studies31, 32 showed statistically significant associations of silent infarction with increasing age. Compared with non-AF strokes, those occurring in the context of AF have a greater mortality and survivors are more likely to suffer a recurrence and greater disability.34 Asymptomatic AF carries the same thromboembolic risk as symptomatic AF. PAF has been shown also to have similar rates of ischaemic stroke and predictors as sustained AF.35, 36
Multivariate analysis from antithrombotic trials in AF have demonstrated several clinical risk factors for stroke in non-rheumatic AF: increasing age, history of hypertension, previous stroke or transient ischaemic attack (TIA) and diabetes.37 A pooled analysis of echocardiographic data from three of these trials demonstrated that left ventricular systolic dysfunction (as defined by global or regional wall motion abnormalities shown on 2D transthoracic echo) is an independent risk factor for stroke in AF.38 Recent 2010 ESC guidance additionally suggests vascular disease and sex category (female) as additional important risk factors (CHA2-DS2-VASc scoring system; see below). Moreover, increased left atrial diameter (measured by m-mode echocardiography) is an independent predictor of thromboembolism.39 The presence of thrombus in the LAA and its precursor, the appearance of spontaneous echo contrast, are also associated with thromboembolism.40 Therefore, patients with AF can be risk stratified for stroke on the basis of clinical and echocardiographic data.
Clinical Manifestations
AF can have a diverse clinical presentation, whether symptomatic or asymptomatic. Patients may report experiencing palpitations, dyspnoea or chest pain. Release of atrial natriuretic peptide (ANP) can be associated with polyuria, although this is relatively uncommon in the elderly. Patients may only present with the consequences of disease: thromboembolic complications, heart failure secondary to the tachycardia-induced cardiomyopathy or symptoms secondary to reduced cardiac output (light-headedness, fatigue). Cognitive impairment secondary to cerebral hypoperfusion or recurrent thromboembolism is important to distinguish as this would identify a potentially treatable cause of impairment. Syncope is not a common presentation of AF and generally indicates additional pathology such as conduction system disease or aortic stenosis.
History and Examination
There needs to be a focused workup concentrating on the following points.
Confirmation of Arrhythmia
The clinician should elucidate any prior history of palpitations and associated symptoms. A review of medication past and present, for warfarin or anti-arrhythmics, should be undertaken and response/tolerance to these agents noted. On examination, there is an irregularly irregular pulse and variation in loudness of the first heart sound. There is good evidence that manual pulse check with ECG follow-up of an irregular pulse is a sensitive screening method.41 A 12-lead ECG is essential, as this can provide evidence of prior myocardial infarction, left ventricular hypertrophy and AV node or bundle branch conduction disease.
Aetiology of Arrhythmia
It is essential to identify any possible reversible triggers that may be responsible for a new episode of AF or deterioration in previously well-controlled disease. An assessment of associated cardiovascular disease should also be made both for aetiology and stroke risk assessment.
Effect of Arrhythmia on the Patient
It is important to identify a clear pattern of symptoms attributable to the arrhythmia and any indication of cardiovascular compromise. Evidence of end-organ effects such as heart failure or stroke should also be sought, as this may influence treatment decisions such as anticoagulation.
Patient Assessment for Management Options
A balanced decision regarding rate versus rhythm control and the risks and benefits of anticoagulation should be made. It is imperative that any potential bleeding risks (such as previous haemorrhage or history of falls) are identified. A social history (emphasizing exercise tolerance, living conditions, access to support and cognitive function) is important. A review of medication will establish potential risks of drug interactions and polypharmacy. Issues of non-compliance should be explored as medication, particularly warfarin, will require stable and consistent administration.
Imaging
Transthoracic echocardiography (TTE) is an essential investigation in any patient with AF, regardless of age. It provides an assessment of the left atrium, mitral valve and left ventricular function and dimensions, thus providing information regarding aetiology and stroke risk assessment. TTE has its limitations since it cannot reliably exclude the presence of thrombus in the LAA. Transoesophageal echocardiography (TOE) is the imaging of choice to examine the LAA for thrombus or its precursor spontaneous echo contrast. Its role in cardioversion is discussed below.
Other Tests
A chest X ray and lung function tests are required when lung disease is suspected. A CT head scan is indicated if there is any evidence of cerebrovascular disease which may be important in making a decision regarding anticoagulation. Relevant blood and urine tests include those to rule out infection or inflammatory disorders, liver and renal functions, thyroid functions, full blood count and coagulation profile.
Management
There are two aims in the management of AF: to prevent thromboembolism and to control the arrhythmia (rate or rhythm control). The classification of AF into paroxysmal, persistent, long-standing persistent and permanent is clinically useful as it gives a clear guide to a management strategy for each patient. In PAF, the aim is to maintain sinus rhythm and control the ventricular rate when AF does occur. For a patient with permanent AF, the decision has been made to accept the arrhythmia and instead symptomatic improvement is attained with ventricular rate control. Persistent AF may presents the clinician with a dilemma: standard practice has been to strive for sinus rhythm by electrical or pharmacological means with symptom control, improved haemodynamics and reduced risk of thromboembolism being the proposed rationale. However, rhythm control medication could pose the problem of pro-arrhythmia and randomized controlled trials42–47 have not shown superiority of rhythm control over rate control; these are summarized in Table 36.3.
Table 36.3 Randomized controlled trials of rate versus rhythm control in AF.
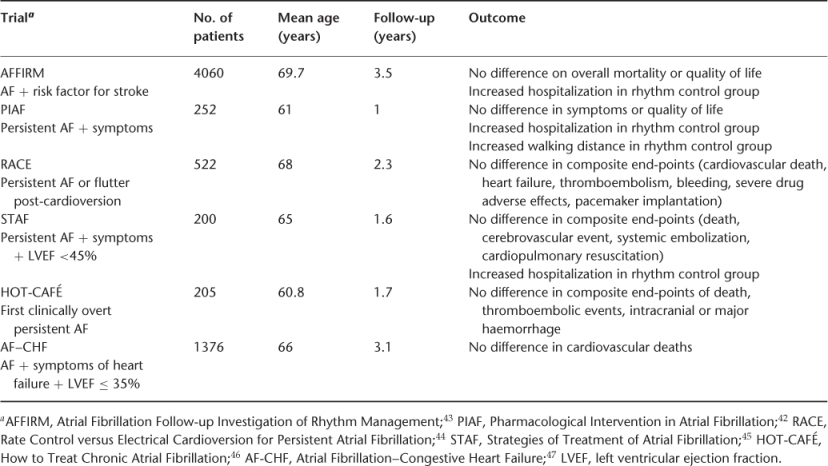
The conclusion to draw from these trials is that for relatively asymptomatic elderly patients with persistent AF, rate control is generally no worse an option than rhythm control. In a first detected episode of AF, one should consider an attempt at rhythm control, cardioversion being the first-line therapy for acute haemodynamic compromise related to AF. However, in elderly patients who are tolerating the arrhythmia, accepting rate control should not be viewed as a failure. Acceptable methods of rate and rhythm control for the elderly are described in the following section. Anticoagulation to prevent thromboembolism needs to be considered whichever strategy is chosen. How a patient is anticoagulated will depend upon the options available, duration of anticoagulation and a risk–benefit analysis for each patient.
Rate Control
As has already been discussed, bradycardia is not a normal finding for the elderly and similarly a slow ventricular rate in an elderly patient with untreated AF implies underlying conduction system disease.48 The aim of rate control is to maintain a patient’s heart rate at what is physiologically appropriate for the level of exertion. This must not be at the expense of symptomatic pauses or bradycardia. Therapy must be tailored to the individual, but rates of 60–80 bpm at rest and 90–115 bpm during moderate exercise have been suggested as a target.49 This can be achieved through medication and/or non-pharmacological methods, although not infrequently no specific rate control therapy is needed in the elderly. The recent RACE II trial50 showed that in patients with fast ventricular rates, but without severe symptoms, stringent rate control (resting heart rate <80 bpm) conferred no symptomatic benefit over lenient rate control (resting heart rate <110 bpm).
Digoxin
Digoxin, a muscarinic agonist, slows AV nodal conduction. This is sufficient to produce adequate rate control for elderly patients with low levels of exertion. This action can be overwhelmed when faced with high sympathetic stimulation, which explains why digoxin is less effective during exercise and why patients with previously well-controlled AF present with inadequate rate control in the context of an acute illness.51
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree