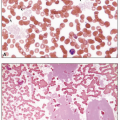
the adequacy of staining looking for uniformity of color: nuclei of leukocytes should appear purple and red cells pink, rather than brick-red or yellow. Review at this low magnification also allows detection of overt abnormalities in cell number, type, and aggregation, and permits finding the optimal areas to evaluate all the blood components. A systematic approach should include evaluation of the lateral and feather edges of blood smears prepared by the wedge method, where a disproportionate distribution in these areas of any large abnormal cells, platelet clumps, and microfilarial parasites, if present, may occur. Red cells are best evaluated in the thin areas of the smear, where they are present in a single layer, closely apposed but not overlapping, and exhibiting normal central pallor. Leukocytes may be best examined in the thick areas of the smear, where many cells are present in a single field of view, but abnormal lymphocyte morphology is best appreciated in the thin areas. Review at higher power (×40 to ×50 objective) allows for an assessment of cell size and a closer examination of the nuclear and cytoplasmic features of individual cells. Oil immersion lenses (×50 and ×100) allow for a detailed evaluation of the quality of the nuclear chromatin and presence of nucleoli, and of cytoplasmic components such as granules, vacuoles, and inclusions. This power provides confirmation of any suspicious cells or organisms seen at lower power. Depending on the procedures defined in each laboratory, the manual differential count consists of identifying and classifying 100, 200, 500, or 1,000 white blood cells and reporting each type as a percentage. Note should also be made of any atypical findings in the leukocytes including abnormal nuclear segmentation and granulation of the neutrophils, or the presence of atypical lymphocytes. The presence in a blood smear of nucleated red cells, megakaryocytes, macrophages, and immature leukocytes, which are normally not found in circulating blood, deserves special note and reporting.
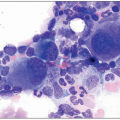
nuclei lacking cytoplasmic features cannot be confidently subclassified. The differential count is performed at high (×40) or oil (×50, ×100) magnification to allow optimal identification of cell types. The count includes nucleated myeloid and erythroid cells in various stages of maturation, promonocytes, monocytes, mast cells, lymphocytes, and plasma cells. Cells that should not be included in the count include macrophages, megakaryocytes, osteoblasts, osteoclasts, stromal, or extrinsic cells. The myeloid to erythroid ratio is calculated by expressing a ratio of all the granulocytes and monocytes and their precursors to the erythroblasts in various maturation stages. Touch preparations should be examined with the same parameters as used for aspirate smears; however, these specimens tend to be characterized by suboptimal morphology and are less reliable.
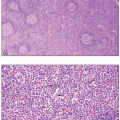
cellularity of the bone marrow specimen. This power provides an opportunity to examine the general pattern, any presence of focal lesions, abnormal cell clusters, and quality of bone structure. Megakaryocyte numbers can be appreciated at low and medium magnification, which also allow for assessment of the relative ratio of myeloid to erythroid cells. Maturation of hematopoiesis is better evaluated at higher magnification (×20 to ×40), which shows the cytology. Maturation of each cell lineage has characteristic features: including increased nuclear segmentation and cytoplasmic granularity in the myeloid lineage, and chromatin condensation and cytoplasmic eosinophilia in the erythroid lineage.
Table 1.1 Hematology reference values in normal adults | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 1.2 Red blood cell characteristics at various ages | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree