Benign Diseases of Leukocytes, Spleen, and Immunoglobulins
Linlin Wang, MD, PhD
Sonam Prakash, MBBS
Benign disorders of white blood cells (WBC) can result in either quantitative or qualitative changes in the WBC. The qualitative changes may be functional and/or morphologic. Both leukocytosis and leukopenia are commonly encountered laboratory findings and can be seen in benign and neoplastic disorders. The clinical findings are extremely helpful in identifying the underlying etiology. One of the first steps is to review the peripheral blood (PB) smear to confirm the leukocyte differential and to evaluate the morphology of each cell type. Further testing is guided by the morphologic findings on the PB smear and on the lineage that is increased or decreased.
The following sections provide a brief overview of benign leukocyte disorders based on the cell lineage that is increased or decreased.
NONNEOPLASTIC DISORDERS OF NEUTROPHILS
The normal reference ranges for WBC counts including relative percentages and absolute cell counts vary by subject age and hospital or outpatient population. Each hospital laboratory must validate reference ranges in its patient population. Total WBC counts are higher in infants, with newborns having the highest WBC count and absolute neutrophil count (ANC) of any age. By the first few weeks of age through early adolescence, lymphocytes become the predominant WBC. This gradually shifts and neutrophils are the predominant WBC in teenagers and adults.1 The neutrophil count also differs by race with individuals of black African descent having lower ANCs.2 Granulopoiesis occurs in the bone marrow and is a closely regulated system of cell proliferation and maturation. The time required for granulopoiesis in the marrow from blasts to neutrophils varies from 1 to 3 weeks. Once released into the peripheral circulation, neutrophils circulate for only a few hours before egressing to tissues. In the PB, there is equilibrium between the circulating pool and the marginated pool of neutrophils adherent to vascular endothelium.
Neutrophilia
Neutrophilia is defined as an ANC that exceeds age-related normal range. The normal reference interval (established for each laboratory separately) is approximately 1.8 to 7.0 × 109/L in adults and 1.0 to 8.5 × 109/L in young children.3 The peripheral neutrophil count only monitors neutrophils in the circulating pool. The primary mechanisms for reactive peripheral neutrophilia are listed in Table 5.1.
Causes of reactive neutrophilia include a broad spectrum of disorders (Table 5.2).4 The most common cause is bacterial infection. Occasional viral infections such as severe acute respiratory distress syndrome or Hantavirus pulmonary syndrome can result in neutrophilia. Another common cause is administration of granulocyte-colony stimulating factor (G-CSF). Other medications including steroids or acute stressful events such as burns or trauma are also not infrequently encountered causes of reactive neutrophilia.
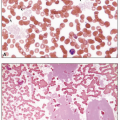
Peripheral smears of reactive neutrophilia, as seen in infections, usually demonstrate toxic granulation, Döhle bodies, and vacuolization (Fig. 5.1A-D). Döhle bodies are pale cytoplasmic inclusions that are parallel stacks of rough endoplasmic reticulum with bound ribosomes. Toxic granulation represents either retained primary granules or altered uptake of stain by secondary granules. Prominent cytoplasmic vacuoles in neutrophils are usually associated with sepsis. Variable degree of left shift in the granulocytic lineage can be seen. In adults with infections, the left shift is usually mild (bands and metamyelocytes) with absent or rare blasts. With peripheral reactive neutrophilia, the bone marrow demonstrates granulocytic hyperplasia with a full spectrum of maturation (Fig. 5.1E). However, the left shift can be prominent in infections in pediatric patients and in patients receiving G-CSF (Fig. 5.2A). In these patients, binucleate neutrophils or very large neutrophils can sometimes be
present and should not be interpreted as dysplasia (Fig. 5.2B, C).5 The hypergranulation induced by G-CSF therapy shows a high density of granules, which stain redder than toxic granulation and often obscure the nucleus (Fig. 5.2D). Left shifted granulocytic maturation can be seen in the marrow with G-CSF administration (Fig. 5.2E).
present and should not be interpreted as dysplasia (Fig. 5.2B, C).5 The hypergranulation induced by G-CSF therapy shows a high density of granules, which stain redder than toxic granulation and often obscure the nucleus (Fig. 5.2D). Left shifted granulocytic maturation can be seen in the marrow with G-CSF administration (Fig. 5.2E).
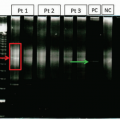
Neutrophilia with left shift can be seen in various hematopoietic neoplasms, especially chronic myeloid leukemia (CML). CML usually has very high WBC (>50 × 109/L) with prominent left shift with a “myelocyte bulge” and associated basophilia (Fig. 5.3).6 Toxic granulation is usually absent. Blasts can be increased. Splenomegaly can be seen in CML. In cases with findings suggestive of CML, molecular or cytogenetic testing for BCR/ABL1 gene rearrangement is warranted. In reactive neutrophilia, WBC count is usually less than 30 × 109/L, except in pediatric patients with infection or patients receiving G-CSF or having tumors that are secreting G-CSF or infections in post-splenectomy states. Basophils are usually not increased. Blasts are very rare, and are usually seen only in patients receiving G-CSF or in pediatric patients. Reactive neutrophilia typically does not cause splenomegaly. Chronic neutrophilic leukemia (CNL) is a myeloproliferative neoplasm that presents with marked leukocytosis (>30 × 109/L) with absolute neutrophilia. In CNL, the peripheral smear does not demonstrate significant left shift (immature granulocytes are <10% of WBC), patients often have splenomegaly, and mutational analysis is positive for CSF3R mutations in over 80% of cases.7
In a patient with absolute neutrophilia, the distinction between reactive and neoplastic etiology (especially CML) requires integration of clinical, hematologic, morphologic, and other laboratory parameters. It is extremely important to carefully review the clinical history for possible reactive etiologies of neutrophilia.
Neutropenia
Neutropenia is defined as ANC in blood below age-related normal range (≤2.5 × 109/L in infants and ≤1.5 × 109/L in all other patient age groups).8 Approximately 25% of healthy children and adults of African ancestry have ANC of 1.0 to 1.5 × 109/L that is considered a normal race-related variant.2 The degree of neutropenia is classified based on ANC; mild neutropenia is defined as ANC of 1.0 to 1.5 × 109/L, moderate neutropenia as ANC of 0.5 to 1.0 × 109/L, and severe neutropenia as ANC below 0.5 × 109/L. Patients with severe neutropenia are at high risk for infections.
Neutropenia can be isolated or associated with abnormalities in other lineages. Most causes of isolated neutropenia are either constitutional or acquired nonneoplastic disorders. In neoplastic processes, neutropenia is often associated with abnormalities in other lineages.
Neutropenia can be transient or chronic. Chronic neutropenia is usually defined as an ANC below 1.5 × 109/L lasting for more than 3 months. Transient isolated neutropenia is usually secondary to infections or certain drugs. These infections include cytomegalovirus, Epstein-Barr virus (EBV), human immunodeficiency virus (HIV), influenza, parvovirus B19, Brucella, paratyphoid, tuberculosis, tularemia, typhoid, Anaplasma phagocytophilum (Fig. 5.4) and other rickettsia, Plasmodium vivax and Plasmodium falciparum. The drugs commonly associated with neutropenia are anticonvulsants (carbamazepine, valproate), antimicrobials (sulfonamides, penicillins, trimethoprim/sulfamethoxazole), antipsychotic (clozapine, olanzapine, phenothiazines), antirheumatics (gold, levamisole, penicillamine), antithyroid (methimazole, propylthiouracil), and other drugs such as aminopyrine, deferiprone, rituximab, and levamisole-adulterated cocaine.9 The mechanisms underlying peripheral neutropenia include proliferation defect, maturation defect, an abnormal distribution, and peripheral destruction (Table 5.3). In practice, more than one of these mechanisms may play a role in a given patient.
The common causes of neutropenia based on age of the patient are listed in Table 5.4. The most common cause of neutropenia in neonates/infants is infection that can be acquired or vertically transmitted such as HIV. Infants can also develop neutropenia due to maternal factors such as hypertension, medications given to the mother and maternal antibodies that cross the placenta and attack fetal neutrophils. Autoimmune neutropenia of infancy usually develops between 1 and 3 years of age. It is not associated with other immune aberrations and usually resolves spontaneously. Congenital neutropenia syndromes are usually recognized during childhood because of associated infections. The clinical, molecular, PB, and bone marrow features of congenital neutropenic disorders are listed in Table 5.5.9, 10, 11, 12, 13 In older children, infection, often viral, is the most common cause of neutropenia.
Cyclic neutropenia is a rare, autosomal-dominant disorder due to mutations in the gene for neutrophil elastase (ELANE or ELA-2) usually confined to exons 4 and 5 in 80% of affected patients.11 These patients have regular oscillations of severe neutropenia lasting for 4 to 6 days that occur with a 21-day periodicity. During periods of profound neutropenia, patients are predisposed to developing painful mouth ulcers, fever, and bacterial infections. The diagnosis of cyclic neutropenia can be established by serial WBC counts at least three times per week for a minimum of 6 weeks to observe at least two neutrophil nadirs. The PB also usually shows relative monocytosis at the nadir of neutropenia. A bone marrow aspiration and biopsy is not required for diagnosis; however, when performed, it demonstrates maturation arrest in myeloid lineage preceding peripheral neutropenia. Management includes the administration of very low amounts of G-CSF shortly before the neutropenic phase of each cycle to counteract the maturation arrest, reduce the nadir, and diminish the cycling. This approach alleviates the symptoms. Cyclic neutropenia is not associated with an increased risk for leukemia or myelodysplasia.
In adults, medication is the most common cause of acquired neutropenia in an outpatient setting and it is important to query this history. In adults, autoimmune neutropenia is often associated with an immune underlying disorder such
as systemic lupus erythematosus (SLE), thyroid disease, or rheumatoid arthritis. The neutropenia is usually self-limited with spontaneous recovery after 2 or 3 years. There may be an increased number of large granular lymphocytes (LGL) in the blood and/or bone marrow. The constellation of neutropenia, splenomegaly, and rheumatoid arthritis is recognized as Felty syndrome. In some of these patients, the LGL proliferation is neoplastic.
as systemic lupus erythematosus (SLE), thyroid disease, or rheumatoid arthritis. The neutropenia is usually self-limited with spontaneous recovery after 2 or 3 years. There may be an increased number of large granular lymphocytes (LGL) in the blood and/or bone marrow. The constellation of neutropenia, splenomegaly, and rheumatoid arthritis is recognized as Felty syndrome. In some of these patients, the LGL proliferation is neoplastic.
Neutropenia can also be seen in myelodysplastic syndromes (MDS). In this setting, neutropenia is often accompanied by abnormalities in other lineages. On the peripheral smear, the neutrophils may show dysplastic features including hypogranularity and nuclear segmentation abnormalities.
Age at onset and duration of neutropenia are critical factors in determining the etiology of neutropenia. Transient neutropenia is common in children and likely secondary to infection. Family history and clinical findings can provide clues to the diagnosis of constitutional neutropenia. In adults, history of infections or medication use, morphologic evaluation of neutrophil morphology, percentage of LGL or other lineage abnormalities on the peripheral smear are helpful in evaluating the etiology of neutropenia.
Neutrophilic Disorders with Abnormal Morphology
Table 5.6 summarizes a list of nonneoplastic disorders presenting with abnormal neutrophil morphology.14 Although these morphologic changes are not specific for a particular etiology, they can provide essential information to guide further evaluation. A detailed clinical history including complete medication list is necessary for evaluation of the underlying etiology.
Functional Defects of Granulocytes
Patients with constitutional neutrophil functional defects are extremely rare but are susceptible to recurrent bacterial and fungal infections. Most constitutional neutrophil function defects are manifested in infancy or early childhood and are listed in Table 5.7.15,16 Acquired neutrophil function defects are much more common but usually not severe. Conditions associated with acquired neutrophil function defects include acute myeloid leukemia (AML), MDS, chronic renal failure, poorly controlled diabetes mellitus, numerous medications (corticosteroid, epinephrine, aspirin, colchicine), autoimmune disorders, chronic infection (e.g., HIV), trauma, surgery, thermal injury, and so forth.
NONNEOPLASTIC DISORDERS OF LYMPHOCYTES
Lymphocytosis
Absolute lymphocytosis is typically defined as a lymphocyte count greater than 4.0 × 109/L in adults and greater than 8.8 × 109/L in children.3 These values provide general ranges; specific upper and lower limits of normal may vary in different laboratories. Lymphocytosis can be either a reactive polyclonal proliferation or a clonal expansion. The distinction between reactive and clonal lymphocytosis is important as it has implications for monitoring and therapy. Clonal lymphocytosis is usually seen as a monotonous population of lymphocytes on the peripheral smear. It will be discussed in a separate chapter.
The causes of benign lymphocytosis are summarized in Table 5.8.17 The most common cause is viral infections, particularly infectious mononucleosis caused by EBV.18 The characteristic morphologic finding is the presence of an increased number of reactive lymphocytes of variable morphology (Fig. 5.14). Correlation with heterophile antibody test is useful in establishing a diagnosis. Many other viruses can cause similar clinical and morphologic features that are referred to as infectious mononucleosis-like syndromes.
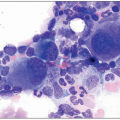
Large granular lymphocytosis can be transient or chronic and can be neoplastic or reactive.19 Reactive etiologies of large granular lymphocytosis are listed in Table 5.9. T-cell large granular lymphocytic (T-LGL) leukemia is a neoplasm that is often associated with neutropenia and by definition is a clonal T-cell process with persistence of the clone for at least 6 months.19 On the PB smear, large granular lymphocytes ( LGL) have a similar morphology in both reactive and neoplastic processes (Fig. 5.16).
Reactive lymphocytosis in most cases is due to an increase in T cells. However, rare etiologies can results in an increased number of polytypic B cells. Persistent polyclonal B lymphocytosis is a syndrome observed in young to middle-aged women with a strong association with cigarette smoking. These patients present with a polyclonal increase in B cells in the PB and binucleate lymphocytes identified on the PB smear. They also show an increase in serum immunoglobulin M (IgM) with or without lymphadenopathy and splenomegaly. Most patients have a stable clinical course on long-term follow-up even though they demonstrate frequent cytogenetic and molecular abnormalities including an extra isochromosome for the long arm of chromosome 3 and BCL2/IGH gene arrangements.20,21
Reactive lymphocytosis commonly demonstrates a spectrum of lymphocyte morphologies including variably sized lymphocytes. In children, lymphocytosis is usually benign with a heterogeneous morphology. If the lymphocytosis is composed of a monotonous population of lymphocytes, flow cytometric immunophenotyping is a useful tool to exclude a neoplastic process.
Lymphopenia
NONNEOPLASTIC DISORDERS OF MONOCYTES Monocytosis
Absolute monocytosis is defined as a monocyte count greater than 1.0 × 109/L.3 Monocytosis can represent a reactive phenomenon or a neoplastic disorder involving monocytes and their precursors. A variety of conditions can raise the monocyte count, including autoimmune disorders (e.g., rheumatoid arthritis, SLE, and inflammatory bowel disease), chronic infections (e.g., mycobacteria, listeria or ehrlichiosis; see Fig. 5.4), underlying solid or hematopoietic tumors, environmental exposure such as alcoholic liver disease, chemotherapy, stem cell transplant, GM-CSF or glucocorticoid administration, hemolytic anemia, cyclic neutropenia, and chronic neutropenia (Fig. 5.17). In neutropenic patients, the monocytosis represents a compensatory phenomenon. The most common cause of reactive monocytosis is chronic infection.
Monocytosis can be transient in settings including post chemotherapy or stem cell transplant, recovery phase of cyclic neutropenia, drug reaction, acute infection, or tissue injury. Chronic monocytosis can be seen in chronic inflammatory disorders, chronic infections, or neoplasms.
In neoplastic disorders, the monocytes are part of the neoplastic clone. Immature monocytes and dysplastic abnormal monocytes can also be present (Fig. 5.18). If monocytosis is persistent (>3 months) and a reactive etiology is not identified, further workup for a neoplastic process such as chronic myelomonocytic leukemia in adults or juvenile myelomonocytic leukemia in children is warranted.
Monocytopenia
Monocytopenia is a decrease in circulating monocytes below the lower reference value of 0.2 × 109/L.3 Although monocytopenia may occur in any nonneoplastic or neoplastic diseases associated with pancytopenia (e.g., acute infections, stress, autoimmune disorders, treatment with glucocorticoids, aplastic anemia, AML, treatment with myelotoxic drugs), a decrease in monocytes is a constant and important feature of hairy cell leukemia. Deficiency or absence of monocytes can also occur in patients with mutations of the hematopoietic transcription factor gene, GATA2. Affected patients sometimes present with nontuberculous mycobacterial infection, especially at cutaneous sites (i.e., MonoMAC syndrome), genital human papillomavirus infection, or lymphedema. These patients are at risk of progression to MDS/AML.22
PANCYTOPENIA
Pancytopenia refers to hemoglobin, WBC count, and platelet count below age/sex-related normal ranges (anemia, leukopenia, and thrombocytopenia). The etiologies/mechanisms of pancytopenia are listed in Table 5.10.23 Constitutional pancytopenia is rare and usually manifests in early infancy; Fanconi anemia is the most common type. Acquired pancytopenia is more common in clinical practice and is often nonneoplastic. Clinical history of infection, nutritional deficiencies, exposure to toxins, autoimmune disorders, and chemotherapy is important for evaluation of pancytopenia. Assessment of morphologic abnormalities of various lineages such as macrocytic anemia or hypersegmentation of neutrophils provides clues to nutritional deficiency (Fig. 5.23). Evaluation of all hematopoietic lineages for dysplasia and presence or absence of circulating neoplastic cells such as blasts, hairy cells, or lymphoma cells is necessary to exclude neoplastic etiologies. If a reactive etiology for pancytopenia is not identified, further workup, including a bone marrow biopsy is helpful to determine the underlying etiology.
NONNEOPLASTIC DISORDERS OF EOSINOPHILS AND BASOPHILS
Eosinophilia
Eosinophilia is defined as an absolute eosinophil count (AEC) more than 0.5 × 109/L independent of age. PB eosinophilia has been divided into mild (0.5-1.5 × 109/L), marked (>1.5 × 109/L), and massive (>5.0 × 109/L) eosinophilia.24 Eosinophilia can be transient, episodic, or persistent (chronic). Persistent eosinophilia is defined as PB eosinophilia recorded on at least two occasions with a minimum time interval of 4 weeks.24
Eosinophilia may be neoplastic or reactive.25,26 The common causes of reactive eosinophilia are listed in Table 5.11. Common causes of eosinophilia are allergic diseases (Fig. 5.24), parasitic infections (Fig. 5.25), and secondary reactions to drugs. Allergic disorders are the most common cause in industrialized nations; parasitic infections are the most common cause of eosinophilia with a history of travel to parasite-endemic areas, and medications/drugs are most common cause in hospitalized patients. Paraneoplastic eosinophilia may be seen in neoplastic diseases including Hodgkin lymphoma, T-cell lymphoma, B-lymphoblastic leukemia, mastocytosis, and carcinomas.
Hypereosinophilia (HE) in the PB is defined as an absolute eosinophil count >1.5 × 109/L and is usually persistent.24 A hypereosinophilic syndrome (HES) is defined by the presence of HE (as defined above), with eosinophil-mediated organ damage and/or dysfunction. It may be neoplastic where eosinophilic expansion is clonal and occurs in the setting of an underlying stem cell, myeloid, or eosinophilic neoplasm; secondary where eosinophilic expansion is driven by overproduction of cytokines by other cell types and is polyclonal; or idiopathic where the underlying cause of HE remains unknown despite extensive etiologic workup.
Basophilia
Basophilia is defined as an absolute basophil count exceeding 0.2 × 109/L.3 Reactive basophilia is very rare. Causes of reactive basophilia include allergic/hypersensitivity disorders, chronic inflammatory conditions, endocrinopathy, renal disease, infections, irradiation, and carcinomas. Basophilia is more commonly seen in association with clonal disorders. The most common cause of basophilia is chronic myelogenous leukemia (Fig. 5.26). Evaluation of granulocytes including the presence of left shift or leukocytosis is important to differentiate neoplastic from reactive etiologies. Basophils frequently degranulate on blood smear that can make their identification difficult.
NONNEOPLASTIC DISEASES OF THE SPLEEN
Nonneoplastic diseases of the spleen include both infectious and noninfectious disorders and can be classified based on the pattern of splenic involvement (Table 5.12). The following section will discuss the salient features of noninfectious and cystic disorders of the spleen.
Reactive follicular hyperplasia can be seen at any age; however, it is more common in children and younger adults. It can be caused by both acute and chronic immunologic stimuli (e.g., bacterial infections, autoimmune diseases including hemolytic processes). It may present with mild to moderate splenomegaly. Morphologically, variably prominent germinal centers with well-defined marginal and mantle zones are present (Fig. 5.27). The germinal centers are positive for CD20, CD10, bcl-6, and negative for bcl-2 immunohistochemical stains. Increased number of plasma cells and small plasma cell aggregates can be identified in the red pulp.27
Reactive lymphoid hyperplasia without germinal center hyperplasia is the most common pattern encountered in viral infections (infectious mononucleosis, herpes simplex virus), transplant recipients, and immunosuppressed individuals (e.g., steroid-treated immune thrombocytopenic purpura, patients with rheumatoid arthritis on methotrexate) and in functional immunodeficiencies encountered in infants and elderly individuals. These patients can present with modest splenomegaly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree