© Springer Science+Business Media New York 2015
Lisa A. Newman and Jessica M. Bensenhaver (eds.)Ductal Carcinoma In Situ and Microinvasive/Borderline Breast Cancer10.1007/978-1-4939-2035-8_1010. Anti-HER2/neu Therapy in DCIS
Amelia Tower1 , Ruta D. Rao3 , Kalliopi P. Siziopikou2, 4 , Melody A. Cobleigh3 and Thomas B. Julian1, 5, 6, 7
(1)
Division of Breast Surgical Oncology, Allegheny General Hospital, 320 East North Avenue, 15212 Pittsburgh, PA, USA
(2)
Robert H. Lurie Comprehensive Cancer Center, Chicago, USA
(3)
Section of Medical Oncology, Department of Internal Medicine, Rush University Medical Center, 1725 West Harrison Street, Suite 809, 60612 Chicago, IL, USA
(4)
Breast Pathology Section, Department of Pathology, Northwestern University Feinberg School of Medicine, 251 East Huron Street, Feinberg 7, 60611 Chicago, IL, USA
(5)
WPAHS Breast Surgical Oncology, Allegheny Health Network, Pittsburgh, USA
(6)
Temple University School of Medicine, Philadelphia, USA
(7)
Medical Affairs, National Surgical Adjuvant Breast and Bowel Project (NSABP), Pittsburgh, USA
Keywords
Ductal carcinoma in situHER2/neu TrastuzumabRadiotherapyBackground/History of HER2/neu
In 1985, three different laboratories simultaneously identified a new gene designated human epidermal growth factor receptor (HER)2, c-erbB2, or epidermal growth factor receptor (EGFR )2. In the years following, the pivotal observation that the HER2 protein is overexpressed in a notable percentage of breast cancers, due to gene amplification, led to a series of functional studies and culminated in the hypothesis that blockade of the signaling pathway would inhibit breast cancer cell growth. These studies resulted in the development of trastuzumab (Herceptin®), a new, targeted therapy for breast cancer [1] .
Today, almost three decades later, anti-HER2-targeted therapy for breast cancer has become a cornerstone for the treatment of HER2-positive disease, with unprecedented success achieved through the use of trastuzumab. When given early in the course of disease, this drug has been shown to have a major impact on patient survival [2]. HER2 is amplified in 15–20 % of invasive breast cancers , and in these cases, the encoded protein is present in abnormally high levels in the malignant cells. Women with breast cancers that overexpress HER2 have an aggressive form of the disease and considerably shortened disease-free survival and overall survival [3]. Some studies have reported higher rates of HER2 overexpression in pure noninvasive ductal carcinoma in situ (DCIS; 56 %), with levels decreasing when invasive cancer is associated with DCIS (22 %) and dropping further in pure invasive cancer (11 %) [4, 5]. In addition, HER2 plays critical roles in the progression of breast cancer tumorigenesis and metastasis [6] .
Many modalities of management of DCIS have mirrored those of invasive breast cancer, and because trastuzumab has proven to be a successful therapeutic agent for the treatment of HER2-positive invasive breast cancers , attention has recently been focused on this drug’s utility in DCIS. This initiative has recently been championed by the National Surgical Adjuvant Breast and Bowel Project (NSABP) in its B-43 clinical trial [7]. In addition, significant focus has been dedicated to investigating the biologic and immunologic effects of trastuzumab in DCIS. As demonstrated by Kuerer et al. in 2010, one intravenous dose of trastuzumab induced immune response in patients with DCIS in the neoadjuvant setting [8] .
The work is just beginning on the use of trastuzumab in DCIS, and the data to date are in their infancy but interesting. Anti-HER2 therapy is a cornerstone of targeted therapy for invasive breast cancer and is a “poster child” for personalized cancer care [1]. In the future, it may also take on that role in the treatment of DCIS.
Pathology of DCIS, with Emphasis on HER2-Positive Lesions
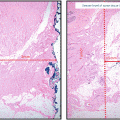
As with invasive breast carcinoma, DCIS encompasses a heterogeneous group of lesions with unique biologic characteristics, a propensity for progression to invasive carcinoma, and variable responses to treatment . The histologic parameters of clinical significance in DCIS include architectural subtypes, nuclear grade, presence or absence of microcalcifications and/or necrosis, estimate of the size/extent of the lesion, and margin status [9–11] .
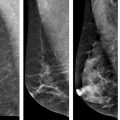
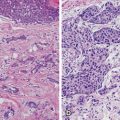
The architectural subtypes of DCIS are classically divided into noncomedo and comedo subtypes; noncomedo subtypes are further subdivided into cribriform, micropapillary, solid, and papillary subtypes, whereas the comedo subtype is defined by high-grade cells, prominent central necrosis, and associated pleomorphic microcalcifications [10–12]. This dichotomous classification of DCIS lesions correlates with a number of differences in many important tumor markers as well: In addition to the high-grade cytology of the cells of the comedo subtype, comedo DCIS lesions are also often negative for estrogen receptor (ER) expression, show frequent amplification of the HER2 gene, are frequently aneuploid, and have mutations in the suppressor gene p53 and high proliferative rates [11–20]. Noncomedo subtypes are composed of cells with low-grade cytology, are very frequently positive for ER and negative for HER2 amplification, are negative for p53 mutations, are not aneuploid, and have low proliferation rates [11–20] . Furthermore, angiogenesis and foci of microinvasion are common around comedo DCIS lesions, whereas noncomedo lesions show low levels of angiogenesis and infrequent microinvasive foci [11, 21–23] . Finally, whereas pathologic correlation of the extent of mammographically detected calcifications is very good for comedo lesions, noncomedo lesions often extend beyond the area of mammographically detected calcifications [11, 24, 25]. This dichotomous behavior also pertains to rates of local recurrence after breast-conserving surgery and radiotherapy, with comedo DCIS showing higher rates of local recurrence than do noncomedo DCIS lesions [11, 23–26].
Reporting of DCIS nuclear grade is a step forward in further subcategorizing DCIS lesions [11, 27–29]. Of the many classification systems that exist, the European system that takes into account the degree of atypia of the nuclei correlates best with clinical outcomes [28, 29]. In this system, the nuclear grade is defined as low (grade 1), intermediate (grade 2), or high (grade 3). This information is now one of the necessary components of a breast pathology report for DCIS, as emphasized in the 2009 College of American Pathologists-American Society for Clinical Oncology (CAP-ASCO) protocol for reporting of DCIS lesions [9] .
Luminal A, luminal B, basal-like, and HER2 subtypes of DCIS along the lines that exist for invasive breast cancers were recently reported by Siziopikou, Livasy, and Bryan [11, 30, 31]. These researchers noted that 61 % of the DCIS lesions they examined belonged in the luminal A group (ER-positive/HER2-negative), 9 % in the luminal B group (ER-positive/HER2-positive), 8 % in the basal-like group (ER-negative/HER2-negative/EGFR-positive/CK5/6-positive), and 16 % in the HER2 group (ER-negative/HER2-positive); 6 % were unclassified [11, 30]. The discovery of these molecular signature subtypes in DCIS further establishes them as precursors of corresponding subtypes of invasive breast carcinomas. HER2 is well known to be expressed in DCIS; its expression is inversely related to ER status. Considerable variability in HER2 expression is reported in DCIS lesions, ranging from 28 to 65 % [32–47]. In most of these studies, HER2 expression was measured by immunohistochemistry (IHC) . However, the numbers of DCIS patients in these studies were limited, ranging from 37 to 255. We recently reported the incidence of HER2 overexpression in an international DCIS study. The cohort included approximately 2500 DCIS patients [48]. In that patient population, the percentage of HER2-positive DCIS cases was much lower (34.9 %) than reported previously among these patients who were candidates for breast preservation. In that study, high-grade DCIS ranged from 81 to 84 % in the two treatment arms. This lower percentage of HER2-positive DCIS cases is much better correlated with the percentage of HER2-positive invasive breast carcinomas, reported to be between 15 and 30 % [3, 37]. Thus, our results seem to support the recent progression model of breast cancer suggesting that high-grade DCIS lesions give rise to high-grade invasive carcinomas, and low-grade DCIS lesions give rise to low-grade invasive carcinomas, a concept supported by recent genetic studies [49]. Of interest, HER2 overexpression is also seen in a small percentage of ER-positive DCIS cases. In a Yale-New Haven Hospital study, 19 % of ER-positive DCIS lesions also over-expressed HER2 [32] . Collins and Schnitt recently calculated that 20 % of newly diagnosed breast cancer cases each year in the USA are DCIS cases (a total of approximately 45,000), and approximately 80 % of those DCIS cases are ER positive (resulting in 36,000 such cases). If 10 to 20 % of these ER-positive DCIS cases also overexpress HER2, then between 3600 and 7200 DCIS cases/year in the USA will be of the ER-positive/HER2-positive phenotype [37, 48]. Knowledge of the status of HER2 overexpression may have potential clinical implications about the use of tamoxifen in this subset of DCIS patients, especially in light of experimental data and data on invasive breast cancer that suggest the simultaneous presence of HER2 overexpression in ER-positive DCIS lesions may interfere with the beneficial effects of tamoxifen in these lesions [49–54]. An understanding of the complex interplay of the molecular pathways that drive the natural history, progression, and response to treatment of DCIS lesions may result in innovative preventive and therapeutic strategies for DCIS .
Trastuzumab
In 1987, the HER2/neu oncogene was described as amplified in 30 % of a sample of 189 primary breast tumors [55]. This study showed the correlation of HER2/neu gene amplification with shortened relapse-free and overall survival. Building on this discovery, a murine monoclonal antibody targeted against HER2/neu was developed [56]. Laboratory trials found that this antibody was effective against the HER2/neu protein, and the humanization of this antibody in 1990 led to its use in clinical trials .
The mechanism by which trastuzumab exerts its therapeutic action remains incompletely understood. Proposed mechanisms include inhibition of HER2 extracellular domain proteolysis, disruption of downstream signaling pathways, G1 cell-cycle arrest, inhibition of DNA repair, suppression of angiogenesis, and potentiation of immune response.
In 1995, based on encouraging phase I and II trial results, a pivotal phase III randomized trial of chemotherapy alone or with trastuzumab for patients with HER2/neu overexpressing, previously untreated, metastatic breast cancer was carried out [3]. This trial showed an improvement in median time to disease progression from 4.6 to 7.4 months (p < 0.001) and an improvement in overall survival from 20.3 to 25.1 months (p = 0.046) with the addition of trastuzumab to chemotherapy. These encouraging results were achieved despite the fact that two thirds of patients in the control group were allowed to receive trastuzumab (with or without chemotherapy) after disease progression on chemotherapy alone. This trial, along with a large phase II supporting trial (Cobleigh et al. [57]), led to the approval of trastuzumab by the US Food and Drug Administration in 1998.
The subsequent use of trastuzumab in the adjuvant setting was based on the results of four large, randomized phase III trials evaluating more than 13,000 women with HER2-overexpressing, early-stage breast cancer [58–61]. The combined analysis of NSABP trial B-31 and North Central Cancer Treatment Group (NCCTG) N9831 showed that the addition of trastuzumab to an anthracycline-and-taxane-based chemotherapy regimen improved both disease-free survival (hazard ratio (HR) 0.48, p < 0.001) and overall survival (HR 0.65, p < 0.001) with a median of 3.9 years of follow-up [58, 59]. Adjuvant trastuzumab was approved for early-stage breast cancer in 2006.
The Herceptin Adjuvant (HERA) trial [62] randomly assigned women to observation or 1 or 2 years of trastuzumab after the completion of standard adjuvant or neoadjuvant chemotherapy. The addition of 1 year of trastuzumab improved disease-free survival (HR 0.76, p > 0.0001) and overall survival (HR 0.76, p = 0.0005) versus observation, but there were no further improvements seen with 2 years of therapy [60]. The Breast Cancer International Research Group (BCIRG) 006 trial [61] confirmed the benefit of trastuzumab when added to anthracycline and taxane-containing chemotherapy regimens (disease-free survival HR 0.64, p < 0.001) as well as to a nonanthracycline regimen of docetaxel, carboplatin, and trastuzumab (TCH; disease-free survival HR 0.75, p = 0.04) [61].
Trastuzumab is currently indicated for the treatment of HER2-overexpressing metastatic breast cancer in combination with paclitaxel for first-line treatment and as a single agent for the treatment of patients who have undergone one or more chemotherapy regimens for metastatic disease. Trastuzumab is indicated for the adjuvant treatment of HER2-overexpressing node-positive or node-negative (ER/progesterone receptor [PR] negative or with one high-risk feature, which includes tumor size > 2 cm, age < 35 years, or tumor grade 2 or 3) breast cancer as part of a treatment regimen consisting of doxorubicin, cyclophosphamide, and either paclitaxel or docetaxel, with docetaxel and carboplatin, or as a single agent following multi-modality anthracycline-based therapy [62].
Risks of Trastuzumab
A reported in metastatic and adjuvant therapy trials [61], trastuzumab is associated with a number of adverse reactions. The incidence of congestive heart failure was increased from 1.3 to 3.2 % when this agent was added to anthracycline-based chemotherapy and was noted to be 0.4 % for patients who received the nonanthracycline-based TCH regimen, although there was no TC control group for comparison. Infusion reactions may include fever, chills, nausea, vomiting, pain (in some cases at tumor sites), headache, dizziness, dyspnea, hypotension, rash, and asthenia. Pulmonary toxicity and exacerbation of chemotherapy-induced neutropenia have been noted. Trastuzumab is classified as pregnancy category D, as it has been linked with fetal harm, including oligohydramnios.
Interaction of Trastuzumab with Radiotherapy
Radiotherapy of patients with breast cancer plays an essential role in local control of the disease and has been shown to reduce recurrence rates by up to 50 % in several studies [63, 64] .
Few studies have explored the relationship between HER2 overexpression and radiosensitivity of breast cancer cells [65]. Preclinical studies have shown that trastuzumab boosts the effectiveness of radiation in xenograft models and in cell lines, without harming irradiated HER2-normal cells [66, 67].
Trastuzumab is used concurrently with radiotherapy in breast cancer patients. The administration of trastuzumab during radiotherapy appears to be safe with regard to cardiac morbidity and mortality, with a relatively modest follow-up duration of less than 5 years. A study by Alanyali et al. [68] showed interactions between radiotherapy and trastuzumab in the HER2-positive breast cancer cell line MDA-MB-453, which were treated with an increased dose of trastuzumab and radiotherapy. Cell viability at 24 and 48 h was statistically significantly decreased (p = 0.0012) compared to single exposures (trastuzumab or radiotherapy), thus indicating that trastuzumab sensitizes HER2-positive breast cancer cells to radiotherapy [68].
Sensitization of human cancer cells to radiotherapy by targeting EGFR family tyrosine kinases is being recognized as a promising novel approach for enhancing the therapeutic effect of radiotherapy [69]. Several studies have reported that treatment of human cancer cells that overexpress the EGFR with either EGFR-blocking monoclonal antibodies (such as cetuximab, also known as Erbitux® or C225) or small-molecule EGFR inhibitors (such as gifitinib, also known as Iressa™ or ZD1839) markedly sensitized the cells to the cytotoxic effect of ionizing radiation both in vitro and in vivo [70–76]. Before evidence of the interaction of trastuzumab with radiotherapy in breast cancer cells, much of this was seen in human head and neck cancer cells. Important preclinical studies with encouraging results prompted clinical trial researchers to investigate the potential enhancement of the therapeutic effects of radiotherapy combined with Erbitux® or Iressa™ treatment in head and neck cancer patients, 90 % of whom have overexpression of the EGF receptor [77]. It has also been reported that trastuzumab sensitizes the cells of head and neck cancer to ionizing radiation [78].
Liang et al. [67] examined the potential role of HER2 in breast cancer radioresistance. They explored whether trastuzumab may sensitize breast cancer cells to ionizing radiation and what may be the major affected downstream pathway responsible for the potential radiosensitization by trastuzumab. That study used a panel of six breast cancer cell lines expressing different levels of HER2 (BT474, SKBR3, MDA453, MCF7, ZR75B, and MDA468). The investigators found that trastuzumab inhibits breast cancer cell proliferation but does not induce apoptosis when used alone; trastuzumab also enhanced radiation-induced apoptosis of the cells in a HER2 level-dependent manner. Liang’s study demonstrated that trastuzumab markedly sensitized the induction of apoptosis by ionizing radiation in cell lines with high levels of HER2, but not in cell lines with low levels of HER2 [7, 67]. Researchers concluded that trastuzumab downregulated the levels of HER2 and reduced phosphorylation levels of specific cells and increased the sensitivity of these cells to radiotherapy [67].
Wattenberg [79] studied radiation’s ability to upregulate monoclonal antibody (mAb) therapy targets. That study used radiation to sensitize tumor cells to antibody-dependent, cell-mediated cytotoxicity (ADCC). Focused on HER2 targeted by trastuzumab, their results showed significant upregulation of HER2 following radiation in 3 of 3 breast cancer cell lines, one of which was triple negative, as well as in residential stem-cell populations. HER2 upregulation was sustained following radiation exposure and was largely dependent on intracellular reactive oxygen species. Improved ADCC and sensitization to the antiproliferative effects of trastuzumab demonstrated the functional significance of radiation-induced HER2 upregulation. That study showed that single-dose radiation enhances mAb therapy. These findings highlight a mechanism for combining radiation with immunotherapy and expand the patient population that can be treated with targeted therapy.
Given the accumulated body of evidence, it is reasonable to ask whether trastuzumab administered during radiotherapy will improve the results of lumpectomy and radiotherapy in women with HER2-positive DCIS. The NSABP B-43 study focuses on this hypothesis, with the hope of better understanding the biology of breast cancer, including its prevention, and of extending the benefits of breast-conserving surgery for women with DCIS [7]. B-43 is examining the potential efficacy and role of postoperative trastuzumab for DCIS in a phase III randomly assigned trial for patients with DCIS treated with breast conservation surgery. Patients are being randomly assigned to whole-breast irradiation with or without concurrent trastuzumab, given in two doses at weeks 1 and 3. The rationale for using trastuzumab concurrently with radiotherapy for HER2-overexpressing DCIS is that trastuzumab only radiosensitizes cells that overexpress HER2, and therefore will enhance the radiation sensitivity of carcinoma more than of surrounding healthy tissues. The primary aim of this clinical trial is to determine whether trastuzumab given concurrently with radiotherapy is more beneficial in preventing subsequent ipsilateral breast cancer recurrence, ipsilateral skin cancer recurrence, or ipsilateral DCIS, when compared with radiotherapy alone in women with HER2-positive DCIS resected by lumpectomy. The secondary aims are to compare the possible benefit of trastuzumab given during radiotherapy to that of radiotherapy alone in preventing subsequent regional or distant recurrence and contralateral invasive or DCIS breast cancer. B-43 will determine if invasive or DCIS disease-free survival, recurrence-free interval, and overall survival can be improved with the addition of trastuzumab to radiotherapy [7].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree