ANGIOTENSIN I-CONVERTING ENZYME
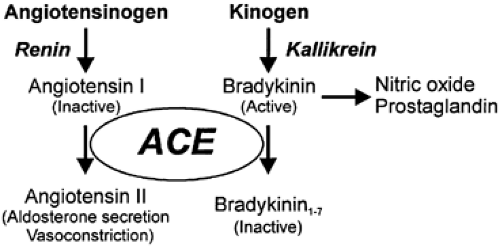
Angiotensin I-converting enzyme (ACE) converts the inactive decapeptide angiotensin I to the active octapeptide angiotensin II (Fig. 79-4). The enzyme is not specific for angiotensin because it cleaves bradykinin, luteinizing hormone-releasing hormone, the enkephalins, and substance P.26,27 It is a more efficient kininase than it is a converting enzyme.27 ACE activates the vaso-constrictor A-II and metabolizes the vasodilator bradykinin (see Fig. 79-4). ACE inhibitors block angiotensin formation and bradykinin degradation. The beneficial effects of ACE inhibitors in the heart and kidney may be a result of both A-II reduction and bradykinin accumulation.
ACE is a metalloenzyme that requires zinc as a cofactor and also needs chloride ions to cleave most substrates.28 Human ACE has a molecular mass of 150 kDa to 180 kDa, of which 146.6 kDa is protein and the remainder is carbohydrate.28 Most ACE is bound to plasma membranes of endothelial cells within vascular tissue. ACE is inserted into the membrane by a 17-amino acid hydrophobic region near the carboxyl terminus (C-terminus hydrophobic anchor peptide).ACE can be released from the plasma membrane by proteolytic cleavage; therefore, some soluble ACE can be detected in blood, urine, edema fluid, amniotic fluid, cerebrospinal fluid, lymph, seminal plasma, and prostate.28,29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree