High-risk type of surgery
Ischemic heart disease
History of congestive heart failure
History of cerebrovascular disease
Insulin therapy for diabetes
Preoperative serum creatinine > 2.0 mg/dl
Heart failure is a major independent predictor of adverse perioperative outcome in noncardiac surgery, even greater than that of ischemic heart disease [4]. These patients should be treated with beta-blockers, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, diuretics, and aldosterone antagonists with perioperative continuation of required therapy. Prior to proceeding with an operation that requires a general anesthetic, clinical and echocardiographic (ECHO) evaluation should be performed in all patients with any type of heart valve disease. For example, severe aortic stenosis, that is, an aortic valve area less than 1 cm2, carries the highest perioperative cardiovascular morbidity and mortality in noncardiac surgery.
Preoperative functional status is probably the most important predictor of perioperative outcome. Low exercise tolerance is associated with poor perioperative outcome [5]. The main purpose of assessment is to predict the individual’s ability to increase oxygen delivery in the perioperative period. Cardiac pulmonary exercise testing (CPET) assesses oxygen uptake and carbon dioxide elimination and can assess both cardiac and respiratory components of the exercise. This is correlated to electrocardiographic (EKG) changes during exercise. Testing for cardiac pathophysiology, such as left ventricular dysfunction, myocardial ischemia, and valve dysfunction, can be primarily evaluated with a thorough history and physical examination. Based upon this, other testing may be required, such as a resting EKG, ECHO, myocardial imaging, cardiac stress tests, and even cardiac catheterization with angiography. However, despite the high technology of several of these studies, they have quite a low overall positive predictive value in the range of 0–33 %.
If coronary angiography reveals abnormalities that will require further intervention such as coronary revascularization, the decision should be made to first address all relevant cardiac issues prior to an operative intervention. Guidelines have been established for the use of beta-blockers prior to a scheduled operation, with the goal of a heart rate of 60–80 beats per minute and a systolic arterial pressure >100 mmHg [5]. In addition to lipid-lowering effects, statins have pleiotropic effects that improve endothelial morphology and function and also stabilize coronary plaques [5]. Angiotensin II inhibitors or angiotensin receptor blockers are also used in these patients. Aspirin taken for secondary cardiac prevention should not be discontinued. Discontinuation of aspirin may be responsible for up to 15 % of all recent acute coronary syndromes in patients with documented stable coronary artery disease.
The combination of aspirin and adenosine diphosphate receptor antagonist therapy (such as clopidogrel, prasugrel, and ticagrelor) plays a significant role in patients with recent placement of coronary stents. Elective surgery should be postponed for at least 6 weeks after placement of a bare-metal stent and for at least 12 months after placement of a drug-eluting stent in order to guarantee a sufficient and adequate endothelialization. Premature discontinuation increases perioperative cardiac morbidity and mortality without significantly reducing the risk of bleeding. If a surgery cannot be delayed for such a long period of time, especially in those with a cancer, aspirin should be continued throughout the peri- and postoperative period.
Optimal preoperative cardiac management includes attention to several factors for a safe outcome. First, the individual stress response, for example, cardiovascular and endocrine, to a given stressor such as a surgical procedure or hematocrit value, must be considered. Secondly, one must address the individual reactions from pharmacological intervention, such as antiplatelet and cardiovascular medications. Third, intra- and postoperative risk factors, such as anemia, hypercoagulability, hypovolemia, inflammatory responses, and cardiovascular depression, must be taken into account [6]. Recognition of such factors and aggressive attempts at appropriate intervention may reduce overall risk more than preoperative management alone (e.g., hemodynamic, endocrine, metabolic, and inflammatory responses, duration of surgery, hypovolemia, hypothermia, pulmonary dysfunction, and pain). Such an approach may render the high-risk patient a lower risk and improve overall outcome [2, 5–7].
Airway difficulties and pulmonary issues have become an increasingly important and difficult area to address. Ventilation of the patient is key, and therefore, a thorough assessment of the patient’s ability to open the mouth and move their head and neck and dentition is a crucial component of the preoperative exam. The Mallampati score is a widely used evaluation system used preoperatively to predict the view of the vocal cords with a laryngoscope blade and the difficulty of intubation [8–12].
The burgeoning phenomenon of surgical patients with an increased body mass index (BMI) has greatly increased the difficulty of airway management and operative approach for both the surgical and anesthetic management teams. Obese patients are complicated from many standpoints. They often have the metabolic syndrome with hypertension and diabetes that have to be medically controlled. Airway management and ventilation are a vital component of their safe care, with many obese patients often requiring such special equipment as fiberoptic bronchoscopy or video laryngoscopy in order to secure an airway. The increasing number of patients who present with sleep apnea has become a major concern for clinicians, with conservative estimates of up to a third of the United States population now have obstructive sleep apnea (OSA). Up to 24 % of males and 9 % of females have mild OSA, with another 11.4 % of males and 4.7 % of females diagnosed with moderate to severe OSA [13–15].
In obese patients, the percentage of OSA increases up to 50 % of men and 30 % of women, and up to 82 % of the men and 93 % of the women are undiagnosed [16, 17]. This diagnosis, whether known or not in a particular patient, has strong implications for the anesthesiologist and surgeon. These patients will have airways that are more difficult to manage, both at the beginning and end of surgery. In addition to often being overweight with a difficult airway, a certain subset is extremely sensitive to anesthetics and narcotics. Their sleep deprivation combined with anesthetic depressant effects can cause them to be sedated for an inordinately long time and even have a critical postoperative respiratory event. Their anesthetic requirements will be decreased and they will need postoperative monitoring of their respiratory and oxygen status to avoid severe respiratory depression and possible arrest secondary to the administration of postoperative pain medications. These patients definitely have a higher rate of postoperative complications and an overall increased morbidity and mortality [18].
The Stop-Bang Questionnaire was developed to help clinicians determine which patients are at a higher risk of sleep apnea [19]. The following table includes the simple eight questions that can be used preoperatively to assess each patient’s risk of sleep apnea (Table 31.2). A high-risk patient may need to be tested preoperatively and taught to use the continuous positive airway pressure (CPAP) machine. They may further require a prolonged course of postoperative monitoring, especially with narcotic use. Many patients do not understand all of the adverse implications that sleep apnea has upon their operative risk and future health. A few such side effects include hypertension, pulmonary hypertension, daytime fatigue, depression, weight gain, diabetes, a compromised immune system, and disruption of circadian rhythms.

Table 31.2
STOP-Bang Scoring Model Questionnaire

Methods
Classically, general anesthesia is the most common method used to care for breast surgical patients. Patients with breast cancer usually come to the operating room for biopsies, mastectomies, axillary node dissections with staging, and possible reconstructions. Whatever their operation, the patients can have many emotional issues that include concerns about the surgery and anesthesia, body image, and prognosis. Anxiety runs high and the anesthesiologist can play a vital role to minimize the psychological stress of the experience.
Anxiolytics given preoperatively, even orally, can help patients feel more comfortable with improved self-control over their situation. If patients have to go to the radiology department for needle localization before surgery, the patient can be given oral sedation for relaxation during the localization procedure. Oral hypnotics that are commonly used include benzodiazepines, such as alprazolam.
A short operative case that is often <1 h in total duration, such as a breast biopsy or lumpectomy, can often be accomplished with local anesthesia and intravenous sedation. Eutectic mixture of local anesthetic (EMLA) with lidocaine and prilocaine can also be used in minor breast surgery without any additional sedation [20]. Intraoperative sedation is often accomplished with a continuous infusion of propofol, an agent that has a short half-life and faster recovery for patients with few side effects, including a low incidence of postoperative nausea and vomiting (PONV) [21].
The anesthetic management has to take many factors into account for the breast surgery patient. General anesthesia is effective and safe, with newer anesthetic agents having a shorter half-life with faster recovery times. When the surgeon is performing a sentinel node biopsy or an axillary node dissection, paralytics should be avoided so that the surgeon is able to identify the functionality and prevent injury to the nerves in this area, particularly the thoracodorsal and long thoracic nerves. General anesthesia with a laryngeal mask airway (LMA) avoids the need for muscle relaxants in these cases, and patients often have less postoperative nausea and vomiting, sore throats, and pain as compared to the use of an endotracheal tube [22].
Thoracic epidural and paravertebral blocks with sedation are becoming more popular in the last several years as their benefits have been increasingly recognized. These include improved postoperative pain relief, decreased narcotic requirements, and a decreased incidence of PONV [23, 24]. Increased patient satisfaction is also a benefit with a trend toward shorter hospital stays [25].
The thoracic paravertebral block is a technique where local anesthetic is injected into the paravertebral space, resulting in an ipsilateral somatic and sympathetic nerve blockade. This block will lead to a unilateral, band-like segmental distribution at the desired levels. It is indicated for the anesthesia and analgesia in patients having a mastectomy, cosmetic breast surgery, and thoracic surgery or in patients with rib fractures [26]. This technique can be done as a single needle injection or as a continuous block by placement of a catheter. The thoracic paravertebral space is a wedge-shaped area that lies on either side of the vertebral column (Fig. 31.1). The anterolateral wall is formed of parietal pleura and the posterior wall is the superior costotransverse ligament. The medial wall is formed from the vertebral body, intervertebral disk, and foramen. This space is continuous with the intercostal space laterally and epidural space medially. A local anesthetic agent can spread longitudinally along this space and even into the intercostal and epidural spaces.
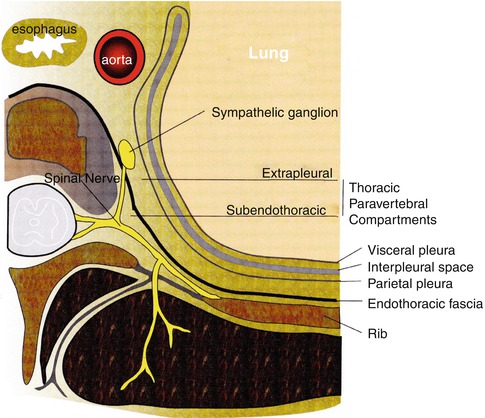

Fig. 31.1
A schematic representation of the thoracic paravertebral space and its structures of relevance to paravertebral block
The spinous processes are the main landmarks for the thoracic paravertebral block, with the C7 spinous process being the most prominent vertebra. Another landmark is the thoracic vertebra, T7, which is at the level of the tip of the scapula. The needle is inserted 2.5 cm lateral to the spinous process, often identifying these landmarks with the guidance of ultrasound (Fig. 31.2) [27–29]. Blockade of T2 through T6 will often be more than adequate for the successful anesthesia of this anatomic distribution. Local anesthetics work directly on the lateral extension of the spinal nerve along with the intercostal nerves and also a medial extension into the epidural space through the intervertebral foramina. This results in the ipsilateral anesthesia that correlates to the desired thoracic dermatome level.
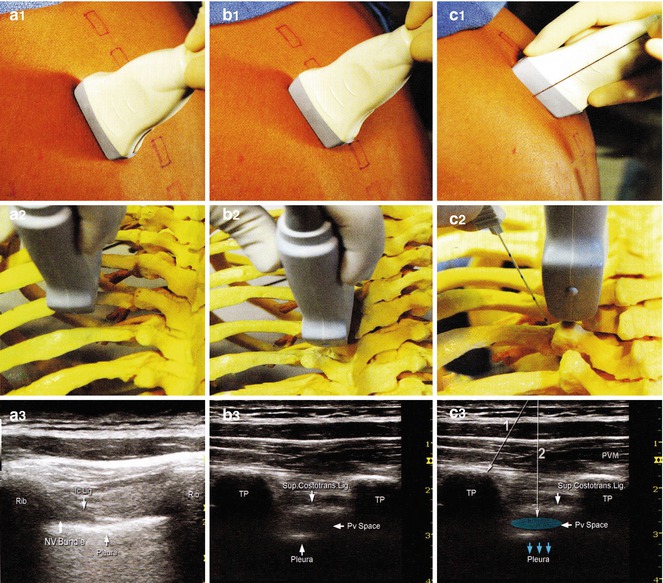

Fig. 31.2
Longitudinal out-of-plane approach to thoracic paravertebral block. The transducer is first placed 5–6 cm lateral to the spinous processes to identify the ribs, parietal pleura, and intercostal spaces (A1 – A3). The transducer is then moved progressively medially to identify transverse processes (B1 – B3). The transverse processes (TP) appear square and deeper than the ribs (round, superficial). The block needle is inserted out of plane to contact the TP (C1–C2 and C3, line 1) and then walked off the TP (C3, line 2) inferior or superior to TP to enter the paravertebral space and for injection of local anesthetic (blue). Proper injection displaces the pleura (blue arrows). PVM paravertebral muscles
The patient can be placed in a sitting, prone, or lateral decubitus position, with the site to be blocked at the uppermost location (Table 31.3) [29]. For a single paravertebral injection, 5–8 ml of local anesthetic can be used at each segmental level to be blocked, or 15–20 ml can be used as a single injection at one level. We utilize ropivacaine 0.5 %, which will provide analgesia for about 8–12 h. Another option is bupivacaine 0.5 % with epinephrine, providing analgesia for up to 18 h. For a continuous block, a bolus injection of local anesthetic, e.g., 8 ml, can be placed and a continuous infusion of ropivacaine 0.2 % or bupivacaine 0.25 % at 10 ml/h started. If a patient-controlled regional analgesia (PCA) system is used, then the continuous infusion can run at 5 ml/h with an as needed bolus of 5 ml every hour. Paravertebral blocks do not result in an extremity motor block and do not impair the patient’s ability to ambulate. This is very helpful in the postoperative setting and overall management of patients. Infection, hematoma, local anesthetic toxicity, nerve injury, total spinal anesthesia, and paravertebral muscle pain are possible complications, albeit quite uncommon.
Table 31.3
Technique of paravertebral block
1. Adequate sedation and monitoring |
2. Sterile technique |
3. Local anesthetic infiltration of subcutaneous tissue and paravertebral muscles |
4. The needle is inserted at 2.5 cm lateral to the spinous process with the intention to contact the transverse process (usually at a depth of 3–6 cm from the skin) |
5. The needle is then withdrawn and redirected superiorly or inferiorly to walk off the transverse process |
6. The needle is then advanced to a depth of 1–1.5 cm past the transverse process |
7. The local anesthetic is then injected after negative aspiration |
(a) Visualization of the needle tip and the control of its path and depth at all times are essential to avoid inadvertent pleural puncture or entry into the intervertebral foramen with epidural spread |
(b) Either a transverse in-line or longitudinal out-of-plane technique can be performed |
A continuous epidural thoracic catheter technique is another alternative for pain control in breast surgery patients, especially for bilateral mastectomies and reconstruction. The major risk factor is a high total spinal level and associated respiratory depression. These patients may need postoperative monitoring to watch for hemodynamic changes, such as hypotension or respiratory complications. There is a risk of possible dural puncture and inadvertent total spinal anesthesia at this high thoracic level. Patients with thoracic epidurals, however, are discharged earlier and have less PONV than patients without them [24].
Postoperative Pain Control
Postoperative pain control can be quite complex in breast surgical patients, often with the need for a combination of different therapeutic approaches in order to maximize the benefit. Regional techniques that include paravertebral blocks and thoracic epidurals are now used to spare narcotic usage and provide for improved postoperative pain control.
For patients with breast lumps or any breast incision, local anesthetic such as bupivacaine 0.25–0.5 % with epinephrine 1:200,000 can be infiltrated into the wound to provide approximately 8–20 h of pain relief. The bupivacaine can be infiltrated preemptively into the area of the surgical incision. In one study, it was shown to decrease intraoperative and postoperative narcotic use and lower postoperative pain scores [30]. This, combined with oral or intravenous ketorolac in appropriate patients, can markedly decrease and often avoid completely the use of narcotics and the risk of PONV [31]. Ketorolac is a nonsteroidal anti-inflammatory agent that provides 4–9 h of pain relief. Its use may be contraindicated in patients with a history of renal insufficiency and gastric bleeding or in geriatric patients. Additionally, there is at least one study that has shown a decrease in the local recurrence rate in breast cancer patients when ketorolac is administered preoperatively [32].
Narcotics are often used for postoperative pain therapy. Patients can be given oral, intravenous, or intramuscular narcotics. Intravenous therapy is most commonly used in the perioperative period and inpatient care. To promote comfort, often a patient-controlled intravenous pump of morphine, hydromorphone, or fentanyl is used. The major drawback is the high incidence of postoperative nausea, vomiting, and drowsiness, and some patients are at an increased risk for respiratory depression.
An On-QTM pain pump is a device that infuses pain medication directly into the wound site and can remain in place for several days postoperatively. A mixture of bupivacaine 0.5 % and ketorolac can be delivered directly to the wound via catheters to provide continuous pain relief. One meta-analysis of surgically placed wound catheters (SPWC) with local anesthetic infusion showed a trend toward improved pain relief and decreased opioid requirements [33].
Intercostal nerve blocks have also been used in patients for minor breast surgeries or for patients with significant comorbidities such as metastatic disease to the lungs [34–36]. The intercostal nerve blocks can be supplemented with an infraclavicular nerve block of the superficial cervical plexus branches that innervate the upper part of the breast and a subcutaneous infiltration of the midline to block the intercostal nerves that cross from the contralateral side [30]. Good outcomes have been reported for both, with decreased postoperative nausea and vomiting with a concomitant decrease in postoperative pain requirements [37].
When a patient has a unilateral or bilateral mastectomy, with or without breast reconstruction, the patient may experience a persistent chronic pain syndrome postoperatively [38]. Postmastectomy pain syndrome is neuropathic pain that persists beyond the normal 3-month healing period. The incidence has been shown to be as high as 52 % [39]. It is seen more commonly in younger patients, up to 65 % of patients [40], and in those who had an axillary lymph node dissection [41] and/or adjuvant radiotherapy [42]. In a recent study, local anesthetic wound infiltration decreased immediate postoperative pain for 90 min in patients undergoing breast cancer surgery. However, this wound infiltration did not appear to reduce the incidence or severity of chronic postoperative pain over the next year in these patients [37].
Regional anesthesia, such as paravertebral blocks or thoracic epidurals, is another method of decreasing the risk of chronic postoperative pain. In one meta-analysis of studies which looked at local anesthetics or regional anesthesia for the prevention of continued pain, the authors concluded that paravertebral blocks may decrease chronic pain after breast cancer surgery in approximately one of every five patients treated [43]. The paravertebral lamina technique performed with continuous catheters can spare opioid use both intraoperatively and postoperatively [23, 44]. Use of local and paravertebral blocks for surgery decreased PONV to 10 % of patients [45].
Postoperative Nausea and Vomiting
Postoperative nausea and vomiting (PONV) is a common and difficult problem to treat. It typically occurs in one third of postoperative patients but can be seen in as many as 80 % of patients. Many breast cancer patients are found to be within the highest risk categories to develop postoperative vomiting. These include the female gender, younger patients, patients with a history of PONV or motion and seasickness, and nonsmokers. Breast surgery itself is a risk factor for increased PONV [46–48].
Hormonal status may also affect the risk of PONV. Estrogen use has been implicated as a risk factor, with one study showing that older patients with estrogen receptor-positive breast cancer had a higher incidence of PONV, possibly due to an altered hormonal milieu [46].
Intraoperatively, both the duration of the operation and the length of the general anesthesia with the use of volatile agents and nitrous oxide were associated with anesthesia-related predictors of PONV [47, 48]. Other predictors include intraoperative and postoperative opioid use, longer-acting narcotics, and larger doses of narcotics [47]. These factors have paved the way for use of regional anesthesia or nonnarcotic alternatives for adequate pain relief.
Different approaches to the problem of PONV are prevention with prophylaxis medications given before or during surgery and the addition of rescue therapy as the symptoms occur. Intravenous treatment options for PONV include serotonin receptor 5HT3 antagonists like ondansetron and granisetron, glucocorticoids such as dexamethasone [49], benzamides such as metoclopramide, butyrophenones (droperidol), and phenothiazines (promethazine). The 5HT3 antagonists are very effective and seem to be somewhat superior to other pharmacological interventions to prevent PONV [50].
The scopolamine transdermal patch is also a great antiemetic agent for narcotic-induced PONV, whether due to intravenous or epidural narcotics. It is often used prophylactically in patients with a history of motion or seasickness that present to the operating room. This patch is effective for up to 3 days and has been shown to be additive to ondansetron in the prevention of PONV [51].
Anesthetic management also has an effect on the risk of PONV. Use of total intravenous anesthesia versus balanced anesthesia with a volatile agent can help prevent PONV [47]. Avoidance of a large dose of neostigmine to reverse muscle relaxation and limitation of narcotic use whether by infiltration of local anesthesia, a regional technique, or use of non-opioid pain medications can also be helpful. A small dose of the hypnotic, propofol, can be used as an antiemetic agent in itself [52].
A multimodal approach to the prevention and treatment of PONV helps to decrease the incidence of complications and increases patient overall satisfaction. Prophylaxis includes assessment of high-risk patients, avoidance of higher-risk agents, and then aggressive use of antiemetic agents. These agents can be given prophylactically or later for treatment as a rescue agent. Routine prophylaxis with ondansetron has been shown to increase patient satisfaction in breast surgery patients [53].
Anesthesia Effects on Outcomes of Breast Cancer Patients
Anesthetic technique and choice of anesthetic agents have been implicated in breast cancer recurrence rates, the development of metastatic disease, and long-term outcome and prognosis [54, 55]. This is a controversial area at present, with some clinicians asking whether the anesthetic management during primary cancer resection can cause, or correlate with, long-term patient outcome. Both the actual surgery and the inhalational anesthetic agents and narcotics can cause immunosuppression after the primary tumor resection. A regional anesthesia technique with decreased use of narcotics and anesthetic agents or use of intravenous agents instead of inhalational agents has been shown in some animal and human studies to decrease the recurrent cancer and metastases rates in oncologic patients [56–58].
A reduction of the surgical stress response and prevention of the decrease in perioperative immune activity could potentially attenuate tumor growth and spread at the time of primary resection of the tumor [59–62]. Surprisingly, this idea that certain anesthetic agents could affect recurrence rates and growth of primary breast cancer was considered more than 30 years ago [63]. At that time, in a study of breast cancer patients, survival rates of patients when halothane was used as the primary anesthetic agent were higher than when ether was used. This was explained by influences of the anesthetic agents both on the pituitary-adrenal cortical system with effects on carcinemia and on the immune system with effects on tumor implantation and metastatic growth. In a more recent study, the type of anesthetic agent used during the surgical procedure was correlated with a decreased risk of recurrence or metastases by fourfold, within the 2.5–4 years of patient follow-up [54]. At present, a clinical multicenter prospective trial has been generated to follow the outcome of patients receiving general versus regional anesthesia [64].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree