Anemia in pregnancy is a global health problem affecting nearly half of all pregnant women worldwide. High fetal demands for iron render iron deficiency the most common cause of anemia of pregnancy, with other micronutrient deficiencies contributing less frequently. In certain geographical populations, human pathogens such as hookworm, malarial parasite and human immunodeficiency virus are important factors in anemia of pregnancy. The hemoglobinopathies, sickle cell disease and thalassemia, represent diverse causes of anemia of pregnancy, requiring specialized care. Aplastic anemia is a rare, morbid cause of anemia of pregnancy and is managed with transfusions until the completion of pregnancy.
“A person, ordinarily in good health … suddenly becomes pale, the surface of the body being waxy and bloodless; she is faint and fatigued; capable of great bodily efforts which, however, produce palpitations and distress; she has pain in the head, impatience of light, throbbing at the temples, and sometimes an universal throbbing, slight confusion in the mind, and a sense of total and extreme prostration. At the same time the pulse is frequent, large, strong and hard; at least, an observer who should not see the pallid face and miserable look of the patient, would pronounce it to be hard…. Still every surface which can be examined during life, is destitute of blood. And after death, the only remarkable appearance is the bloodlessness of the tissues.” Walter Channing, 1842
In 1842, Walter Channing, Dean of Harvard Medical School, published the first documented account of puerperal anemia in his narrative of Mrs H, who developed a fatal nonhemorrhagic anemia shortly after delivery. Addison soon followed with his report of idiopathic anemia, later renamed pernicious anemia owing to its grim prognosis, with the term “pernicious anemia of pregnancy” coined to describe the Channing disease. Landmark advances in the early part of the twentieth century uncovered the pathologic basis of classic pernicious anemia, demonstrating the therapeutic potential of parenteral liver extract and the central role of gastric intrinsic factor in cobalamin (vitamin B 12 ) absorption. Pernicious anemia of pregnancy was shown to be primarily caused by a deficiency of folate rather than vitamin B 12 , based on the observations that affected women lacked gastric achlorhydria and exhibited a slow or absent response to liver supplementation.
Anemia of pregnancy is currently recognized as a major global health problem, affecting nearly half of all pregnant women worldwide. The pernicious anemia of Channing, once regarded as a model for puerperal and gestational anemia, has been shown to be rare, accounting for only 1 of 200 to 1 of 1000 cases. Hydremia of pregnancy, a gestational decrease in hemoglobin levels because of a disproportionate increase in plasma volume, is the major physiologic contributor to anemia of pregnancy. Globally, the most important pathologic cause of anemia of pregnancy is iron deficiency, arising as a consequence of increased fetal use of iron. In nonindustrialized countries, hookworm, malarial parasite, human immunodeficiency virus (HIV), and deficiencies in folate and other micronutrients may contribute to anemia of pregnancy. Pregnancy-associated complications, including sepsis, infection, preeclampsia, malignancy, marrow failure, can also precipitate anemia.
Anemia of pregnancy: definitions and epidemiology
The World Health Organization (WHO) defines anemia of pregnancy as a hemoglobin level of less than 11 g/dL, or hematocrit less than 33%, at any point during pregnancy. The US Centers for Disease Control and Prevention (CDC) defines anemia of pregnancy as a hemoglobin level of less than 11 g/dL, or hematocrit less than 33%, in the first or third trimester or hemoglobin less than 10.5 g/dL, or hematocrit less than 32%, in the second trimester.
- •
Anemia of pregnancy primarily affects women of low socioeconomic status . Globally, by WHO criteria, 52% of pregnant women from undeveloped or developing countries are anemic compared with 20% from industrialized nations. The highest prevalence is among pregnant women in India (88%), followed by Africa (50%), Latin America (40%), and the Caribbean (30%).
- •
The risk of anemia of pregnancy increases with progression of pregnancy . By CDC criteria, among low-income pregnant women in the United States, 8% are anemic in the first trimester, 12% in the second, and 34% in the third. The prevalence of third-trimester anemia is viewed by the US Department of Health and Human Services (DHHS) as a major indicator of reproductive health among low-income women, with the highest prevalence in African Americans (48.5%), followed by American Indians and Alaska Natives (33.9%), Hispanics and Latinas (30.1%), Asians, Native Hawaiians, and other Pacific Islanders (29%), and Whites (27.5%). The Healthy People 2010 initiative, launched in 2000 by the DHHS, proposed to reduce the prevalence of third-trimester anemia among low-income women to 20% or less during the subsequent decade ; however, as of 2008, the overall prevalence had increased compared with previous years, with only 1 state achieving DHHS goals (Montana, with 15.4% of low-income pregnant women classified as anemic in the third trimester).
Iron homeostasis
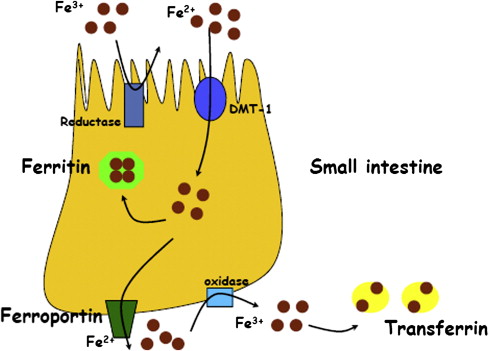
Dietary iron is absorbed by intestinal epithelial cells in the duodenum and jejunum. Absorption depends on gastric acid, which maintains iron in its soluble ferrous (Fe 2+ ) form rather than the insoluble ferric (Fe 3+ ) form. Intracellular iron within macrophages is liberated from senescent erythrocytes during erythrophagocytosis. Iron from both intestinal epithelial cells and macrophages is transported, via ferroportin channels, to the circulation, where it is bound to serum transferrin, which carries the bound iron to target cells ( Fig. 1 ). Transferrin receptors on the surfaces of erythroid progenitors, lymphocytes, and other proliferating cells bind and internalize the transferrin-iron complex, releasing iron intracellularly through the transferrin cycle. Ferritin, an intracellular protein that binds and sequesters iron, is leaked into the circulation in small levels; serum ferritin levels are an accurate indicator of total body iron stores, although the function of intracellular ferritin is unknown.
The primary regulator of iron use is hepcidin, which binds ferroportin and induces its endocytosis and degradation in lysosomes. High hepcidin levels cause intracellular accumulation of iron and impairment of iron use, as seen in anemia of inflammation; by contrast, hepcidin deficiency, seen in hemochromatosis, results in uncontrolled release of iron into the circulation. Transferrin, ferritin, and hepcidin are produced by the liver. The latter 2 are acute phase reactants, and their levels may be elevated during infection, inflammation, or stress.
Iron Requirements During Pregnancy
Approximately 1190 mg of iron is required to sustain pregnancy from conception through delivery. Processes such as maternal and fetal erythropoiesis, as well as blood loss during labor and delivery, consume iron, whereas cessation of menses during pregnancy and lactation conserve iron. The net iron balance during pregnancy, delivery, and the postpartum period is a deficit of 580 mg. When averaged over a gestational period of 290 days, the overall maternal iron deficit amounts to an iron requirement of 2 mg/d, in addition to the recommended daily allowance of 15 mg for women of childbearing age. By comparison, the average daily iron absorption from Western diets is 1 to 2 mg/d, or 3 to 5 mg/d from diets rich in iron-containing foods. Most women therefore cannot optimally maintain iron needs during pregnancy even under optimal dietary circumstances, accounting for the high incidence of pregnancy-associated anemia in nonindustrialized countries, where nutritional status is poor.
The kinetics of iron use over the course of pregnancy gives rise to predictable fluctuations in hematologic parameters :
- •
Maternal hemoglobin levels decline progressively from the first to the ninth month of gestation, then increase in the month before delivery. In the absence of iron supplementation, the nadir is approximately 10.5 g/dL at 27 to 30 weeks of gestation. Iron supplementation alters this kinetics, producing a less-severe nadir (11.5 g/dL) occurring earlier (22–26 weeks).
- •
Serum ferritin levels decline beginning in the third trimester, then increase in the month before delivery owing to the acute phase response. The nadir is approximately 15 g/μL at 35 to 38 weeks in the absence of iron supplementation or 20 μ/L at 31 to 34 weeks with iron.
- •
Red cell mean corpuscular volume (MCV) may decline during the third trimester, although the magnitude of the effect is mitigated by iron supplementation.
- •
Serum erythropoietin levels increase steadily during the first and second trimesters, then surge in the third trimester. In both pregnant and nonpregnant patients, erythropoietin secretion, mediated by the kidneys, is inversely proportional to hemoglobin levels and blood oxygen content. In pregnancy, iron supplementation may lower peak erythropoietin levels.
- •
Maternal hepcidin levels are reduced at term to facilitate iron transfer and use. Women with iron deficiency in pregnancy have more profound levels of hepcidin suppression at term than those in iron-replete women, although interpretation of such findings is complicated by hepcidin’s role as an acute phase reactant, with increased levels at the time of labor.
Hemodynamic Changes During Pregnancy
Hydremia of pregnancy was first proposed by German and French physicians in the 1830s and formally demonstrated in 1934 by Dieckmann and Wegner, who measured plasma volume, red cell mass, and hemoglobin levels at intervals throughout pregnancy and observed that although all 3 parameters increased with gestation, the increase in plasma volume was greater than that of red cell mass or hemoglobin levels. As a general rule, the total circulatory volumes during pregnancy increase by a factor of 30% to 50% compared with baseline, although in individual studies, considerable variation is reported, reflecting differences in study populations and in techniques used to measure plasma and cell volumes. During pregnancy, the following specific hemodynamic changes occur:
- •
Plasma volume decreases in the first 6 weeks of gestation, then expands from the sixth week to 34 to 36 weeks, achieving levels 50% above baseline.
- •
Red cell mass decreases in the first 12 weeks, then increases from 12 weeks to the third trimester, reaching levels that are 20% to 30% above baseline.
- •
Both plasma volume and red cell mass decline in the final month of gestation, returning to prepregnancy levels by 6 to 8 weeks post partum.
The increase in maternal circulatory volume during pregnancy is accompanied by a decrease in systemic vascular resistance and an increase in perfusion of the uteroplacental and renal vascular beds, thought to be the effect of multiple hormones (eg, renin, angiotensin II, aldosterone, thromboxane A 2 , cortisol, estrogens, progesterone, antidiuretic hormone, atrial natriuretic hormone, and prostacyclin). Maternal expansion of plasma volume and red cell mass supports fetal amniotic fluid production, mitigates hemodynamic insults such as hemorrhage, enhances total blood oxygen binding capacity, and facilitates tissue oxygen delivery. The importance of maternal circulatory expansion is illustrated by the consequences of hypovolemia, which include reduction in maternal glomerular filtration rate and development of fetal oligohydramnios.
Iron homeostasis
Dietary iron is absorbed by intestinal epithelial cells in the duodenum and jejunum. Absorption depends on gastric acid, which maintains iron in its soluble ferrous (Fe 2+ ) form rather than the insoluble ferric (Fe 3+ ) form. Intracellular iron within macrophages is liberated from senescent erythrocytes during erythrophagocytosis. Iron from both intestinal epithelial cells and macrophages is transported, via ferroportin channels, to the circulation, where it is bound to serum transferrin, which carries the bound iron to target cells ( Fig. 1 ). Transferrin receptors on the surfaces of erythroid progenitors, lymphocytes, and other proliferating cells bind and internalize the transferrin-iron complex, releasing iron intracellularly through the transferrin cycle. Ferritin, an intracellular protein that binds and sequesters iron, is leaked into the circulation in small levels; serum ferritin levels are an accurate indicator of total body iron stores, although the function of intracellular ferritin is unknown.
The primary regulator of iron use is hepcidin, which binds ferroportin and induces its endocytosis and degradation in lysosomes. High hepcidin levels cause intracellular accumulation of iron and impairment of iron use, as seen in anemia of inflammation; by contrast, hepcidin deficiency, seen in hemochromatosis, results in uncontrolled release of iron into the circulation. Transferrin, ferritin, and hepcidin are produced by the liver. The latter 2 are acute phase reactants, and their levels may be elevated during infection, inflammation, or stress.
Iron Requirements During Pregnancy
Approximately 1190 mg of iron is required to sustain pregnancy from conception through delivery. Processes such as maternal and fetal erythropoiesis, as well as blood loss during labor and delivery, consume iron, whereas cessation of menses during pregnancy and lactation conserve iron. The net iron balance during pregnancy, delivery, and the postpartum period is a deficit of 580 mg. When averaged over a gestational period of 290 days, the overall maternal iron deficit amounts to an iron requirement of 2 mg/d, in addition to the recommended daily allowance of 15 mg for women of childbearing age. By comparison, the average daily iron absorption from Western diets is 1 to 2 mg/d, or 3 to 5 mg/d from diets rich in iron-containing foods. Most women therefore cannot optimally maintain iron needs during pregnancy even under optimal dietary circumstances, accounting for the high incidence of pregnancy-associated anemia in nonindustrialized countries, where nutritional status is poor.
The kinetics of iron use over the course of pregnancy gives rise to predictable fluctuations in hematologic parameters :
- •
Maternal hemoglobin levels decline progressively from the first to the ninth month of gestation, then increase in the month before delivery. In the absence of iron supplementation, the nadir is approximately 10.5 g/dL at 27 to 30 weeks of gestation. Iron supplementation alters this kinetics, producing a less-severe nadir (11.5 g/dL) occurring earlier (22–26 weeks).
- •
Serum ferritin levels decline beginning in the third trimester, then increase in the month before delivery owing to the acute phase response. The nadir is approximately 15 g/μL at 35 to 38 weeks in the absence of iron supplementation or 20 μ/L at 31 to 34 weeks with iron.
- •
Red cell mean corpuscular volume (MCV) may decline during the third trimester, although the magnitude of the effect is mitigated by iron supplementation.
- •
Serum erythropoietin levels increase steadily during the first and second trimesters, then surge in the third trimester. In both pregnant and nonpregnant patients, erythropoietin secretion, mediated by the kidneys, is inversely proportional to hemoglobin levels and blood oxygen content. In pregnancy, iron supplementation may lower peak erythropoietin levels.
- •
Maternal hepcidin levels are reduced at term to facilitate iron transfer and use. Women with iron deficiency in pregnancy have more profound levels of hepcidin suppression at term than those in iron-replete women, although interpretation of such findings is complicated by hepcidin’s role as an acute phase reactant, with increased levels at the time of labor.
Hemodynamic Changes During Pregnancy
Hydremia of pregnancy was first proposed by German and French physicians in the 1830s and formally demonstrated in 1934 by Dieckmann and Wegner, who measured plasma volume, red cell mass, and hemoglobin levels at intervals throughout pregnancy and observed that although all 3 parameters increased with gestation, the increase in plasma volume was greater than that of red cell mass or hemoglobin levels. As a general rule, the total circulatory volumes during pregnancy increase by a factor of 30% to 50% compared with baseline, although in individual studies, considerable variation is reported, reflecting differences in study populations and in techniques used to measure plasma and cell volumes. During pregnancy, the following specific hemodynamic changes occur:
- •
Plasma volume decreases in the first 6 weeks of gestation, then expands from the sixth week to 34 to 36 weeks, achieving levels 50% above baseline.
- •
Red cell mass decreases in the first 12 weeks, then increases from 12 weeks to the third trimester, reaching levels that are 20% to 30% above baseline.
- •
Both plasma volume and red cell mass decline in the final month of gestation, returning to prepregnancy levels by 6 to 8 weeks post partum.
The increase in maternal circulatory volume during pregnancy is accompanied by a decrease in systemic vascular resistance and an increase in perfusion of the uteroplacental and renal vascular beds, thought to be the effect of multiple hormones (eg, renin, angiotensin II, aldosterone, thromboxane A 2 , cortisol, estrogens, progesterone, antidiuretic hormone, atrial natriuretic hormone, and prostacyclin). Maternal expansion of plasma volume and red cell mass supports fetal amniotic fluid production, mitigates hemodynamic insults such as hemorrhage, enhances total blood oxygen binding capacity, and facilitates tissue oxygen delivery. The importance of maternal circulatory expansion is illustrated by the consequences of hypovolemia, which include reduction in maternal glomerular filtration rate and development of fetal oligohydramnios.
Iron deficiency in pregnancy
Clinicians long recognized that hydremia alone could not account for the hemoglobin levels of less than 7 g/dL in 10% to 70% of pregnant women reported in the early twentieth century studies. A central role for iron deficiency in anemia of pregnancy was demonstrated in the 1950s by the frequent finding of hypochromia, microcytosis, and anisocytosis in the blood smears of pregnant women with anemia, and the resolution of such abnormalities following iron supplementation. Iron deficiency has since been recognized as the most common cause of anemia of pregnancy worldwide, manifesting predominantly in the third trimester, when iron is maximally accumulated to accommodate erythropoiesis in the growing fetus.
Risk Factors
In addition to poor nutritional intake, factors that impair iron absorption may precipitate iron deficiency in pregnancy, including bariatric surgery, antacids, and deficiencies of micronutrients such as vitamin A, vitamin C, zinc, and copper.
Clinical Manifestations
Fatigue, pallor, light-headedness, tachycardia, dyspnea, poor exercise tolerance, and suboptimal work performance have all been reported in pregnant or postpartum women with iron deficiency, as have postpartum depression, poor maternal/infant behavioral interactions, impaired lactation, low birth weight, premature delivery, intrauterine growth retardation, and increased fetal and neonatal mortality. Although supplemental iron improves the hematologic abnormalities of iron deficiency in pregnancy, the therapeutic benefits to neonatal mortality, infant morbidity, and child development are uncertain.
Diagnosis
By either the CDC or WHO criteria, the presence of anemia in combination with a low ferritin level (<15–20 μg/L) is considered diagnostic of iron deficiency in pregnancy. Diagnosis is complicated by the increase in ferritin levels during the third trimester, when iron deficiency is most likely to be present. If ferritin levels are normal or elevated, hypochromia, microcytosis, or a reduced red cell MCV may support a picture of iron deficiency. C-reactive protein is an alternate measure of inflammation; a normal or elevated ferritin level with a normal C-reactive protein level should prompt investigation for alternate causes of anemia, such as hemoglobinopathies. Soluble transferrin receptor (sTfR) concentration, released into the circulation by transferrin receptor–expressing cells, correlates inversely with total body iron content and exhibits high sensitivity and specificity in the diagnosis of iron deficiency during pregnancy. Because sTfR is not an acute phase reactant, its levels do not increase with inflammation, although they can be influenced by red cell mass or erythropoietic activity and are often low in early pregnancy, when erythropoiesis is reduced.
Prevention
The WHO, CDC, and US Food and Drug Administration (FDA) recommend that all pregnant women receive oral iron supplementation from the beginning of gestation to 3 months post partum, at doses of 27 mg/d (FDA), 30 mg/d (CDC), or 60 mg/d (WHO), although doses as low as 20 mg/d may be effective. The major side effects of oral iron supplementation are gastrointestinal symptoms, which occur in a dose-dependent manner, primarily at doses of 200 mg/d or more.
Treatment
Patients with mild anemia (hemoglobin level, 9.0–10.5 g/dL) should receive oral iron at 160 to 200 mg of elemental iron daily, with an expected increase in hemoglobin levels of 1 g/dL after 14 days of therapy. Compared with oral iron, parenteral iron demonstrates faster hematologic recovery, likely because of variations in oral iron tolerability, absorption, and compliance. Parenteral iron may be administered in the second or third trimester to patients who have moderate to severe anemia (hemoglobin <9 g/dL), who are intolerant to oral iron, or who fail to respond appropriately to oral therapy. Four preparations of parenteral iron are available in the United States: iron dextran (low–molecular-weight InFeD and high–molecular-weight DexFerrum), sodium ferric gluconate (Ferrlecit), iron sucrose (Venofer), and ferumoxytol (Feraheme). Among pregnant women who received parenteral iron in published studies, 15% to 20% developed thrombosis, although the relationship between these factors is uncertain. Administration of recombinant human erythropoietin, in combination with parenteral iron, may be an alternate therapy for pregnant women with anemia, who are refractory to oral iron. Darbepoietin has been similarly used in a single case report of a pregnant woman with chronic kidney disease. Larger studies are needed to confirm the appropriateness, safety, and feasibility of erythropoietic stimulating agents in anemia of pregnancy.
Other nutritional deficiencies in anemia of pregnancy
Folate and Cobalamin Deficiency
Folate deficiency is historically regarded as the second most common cause of anemia of pregnancy after iron deficiency, although in many modern series vitamin B 12 deficiency may be more prevalent, particularly in underprivileged areas. In studies from India, Turkey, Africa, Newfoundland, and Venezuela, 10% to 100% of pregnant women have a diagnosis of folate deficiency (defined as a serum level of <2.5–3.0 ng/mL), whereas 30% to 100% have vitamin B 12 deficiency (defined as a serum level of <160–200 pg/mL). The prevalence of folate or vitamin B 12 deficiency increases with gestation.
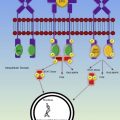
Fetal growth depends on folate and vitamin B 12 because both are involved in the synthesis of tetrahydrofolate, an integral component of deoxyribonucleic acid synthesis and nuclear maturation ( Fig. 2 ). Dietary folate is absorbed in the jejunum; poor nutrition and intestinal malabsorption can precipitate folate deficiency in pregnancy. Oral vitamin B 12 is absorbed in the ileum; R proteins (eg, haptocorrins, secreted by the salivary gland) bind vitamin B 12 in the acid environment of the gastrium, transporting vitamin B 12 to the duodenum where pancreatic proteases degrade the R proteins, releasing vitamin B 12 for binding by intrinsic factor (from gastric parietal cells) and subsequent uptake by ileal enterocytes. Malabsorption, ileal resection, intestinal parasites, atrophic gastritis, antihistamines, and proton pump inhibitors can all increase the risk of vitamin B 12 deficiency in pregnancy.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree