This article reviews pregnancy outcome in women diagnosed with a myeloproliferative neoplasm (MPN), and discusses possible risk markers and the pathogenesis of poor pregnancy outcome. An outline of the key factors regarding the diagnosis and management of MPN in women of reproductive potential is followed by a description of the authors’ management strategy for standard and high-risk pregnancy in MPN patients.
The myeloproliferative neoplasms (MPNs), previously known as the myeloproliferative disorders, are generally indolent stem cell malignancies, characterized by a propensity to thrombotic or hemorrhagic events and, less frequently, transformation to myelofibrosis or acute myeloid leukemia. The MPNs defined by the World Health Organization (WHO) encompass the more commonly encountered essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF), and the rarer entities MPN unclassified, MPN syndromes overlapping with myelodysplasia, mast cell disorders, chronic neutrophilic leukemia, and hypereosinophilic syndrome. The combined incidence of ET, PV, and PMF is approximately 6 in 100,000 to 9 in 100,000, with a peak frequency between 50 and 70 years. However, for ET in particular there is a second peak in women of reproductive age, and 15% of patients with PV are younger than 40 years at the time of diagnosis. Thus these diseases are encountered in women of reproductive potential, and may indeed be diagnosed in pregnancy or during investigation of recurrent pregnancy loss or infertility. Historical case reports of pregnancy and retrospective case series, albeit likely to be subject to reporting bias, suggest significant maternal morbidity and poor fetal outcome. Pregnancy in these conditions is clearly complicated, and a proportion of women with MPN will require disease-specific intervention in pregnancy.
Pathogenesis of MPN
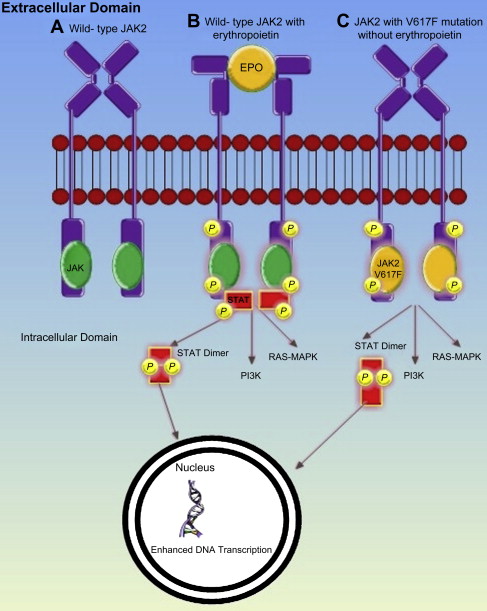
MPNs result from the transformation of a hemopoietic progenitor cell, characterized by overproduction of mature blood cells. In 2005, 4 groups simultaneously reported the discovery of a point mutation in exon 14 of Janus Kinase 2 (JAK2V617F), where phenylalanine is substituted by valine, which enables constitutive activation of JAK 2. Both wild-type and mutant JAK2 bind to the intracytoplasmic tail of many common hemopoietic cytokine receptors, which include those cognate receptors for erythropoietin, thrombopoietin (TPO), and granulocyte colony stimulating factor. When the ligand binds to one of these receptors, phosphorylation of JAK2 and the receptor itself occurs, initiating signaling via the JAK-STAT, MAPK, and PI3K pathways. This process is independent of the presence of ligand receptor interaction in the presence of JAK2V617F ( Fig. 1 ). The JAK2V617F mutation is present in 95% of patients with PV and 50% of patients with ET or myelofibrosis. Many of the remaining patients with JAK2V617F-negative PV have a mutation in exon 12. A proportion of patients with ET or myelofibrosis have one of several mutations of the transmembrane domain of the TPO receptor cMPL, which also causes constitutive activation. All these mutations have been shown to produce an MPN-like phenotype in murine models.
Pregnancy outcome data and risk factors for MPN
ET is the commonest MPN in women of childbearing age, and a significant number of cases of pregnancy have been reported in the literature, but these data are insufficient to devise evidence-based management guidelines. A meta-analysis reported the outcome of 461 pregnancies in women diagnosed with ET. The live birth rate was 50% to 70%; first trimester loss occurred in 25% to 40% and late pregnancy losses in 10%. Rates of placental abruption (3.6%) and intrauterine growth restriction (IUGR) (4.5%) were higher than in the general population. Postpartum thrombotic episodes were reported in 5.2% of pregnancies and pre-/postpartum hemorrhage in 5.2%. A summary of 208 historical cases of ET collated from case series that included greater than 6 pregnancies produced comparable data ( Table 1 ). The literature for pregnancies affected by PV is sparse; pregnancy outcome in the authors’ own case series of 18 pregnancies in PV combined with 20 historical reports was concordant with the pregnancy outcomes in ET, and is summarized in Table 2 . In PV first trimester loss was the most frequent complication (21%), followed by late pregnancy loss (18%), IUGR (15%), and premature delivery (13%), which included 3 neonatal deaths resulting in a 50% survival rate. Maternal morbidity was also significant including 3 thromboses, 1 large postpartum hemorrhage, 4 cases of preeclampsia, and 1 maternal death associated with evidence of a deep vein thrombosis, pulmonary emboli, sagittal sinus thrombosis, and disseminated intravascular coagulation. The literature regarding PMF is more limited. A report of 4 pregnancies in PMF combined with 4 historical cases suggested a 50% risk of fetal loss; no maternal complications of thrombosis or disease progression were noted, but the numbers are probably too small to draw any firm conclusions ( Table 3 ).
| Author Ref. | Patients, n | Pregnancies, n | Maternal Outcome | Live Birth Total | Pregnancy Loss Total | Loss <12/40 | Loss >12/40 | IUGR | Placental Abruption | Live Birth Premature Delivery <37/40 | Live Birth FTD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Belluci et al | 3 | 11 | Detail not available | 4 | 7 | 6 | 1 | Detail not available | 1 | 2 | 2 |
| Beard et al | 6 | 9 | 1 phlebitis, 1 leg ulcer, 1 PPH | 8 | 1 | 1 | 0 | 0 | 0 | 1 | 7 |
| Leone et al | 8 | 10 | Detail not available | 7 | 3 | 0 | 3 | Detail not available | 0 | 0 | 7 |
| Pagliaro et al | 9 | 15 | 2 VTE, 2 TIA, 1 hemorrhage | 9 | 6 | 4 including 1 TOP | 2 | 2 | 0 | 4 | 5 |
| Randi et al | 13 | 16 | 3 VTE | 13 | 3 | 3 | 0 | 0 | 0 | 3 | 10 |
| Cincotta et al | 12 | 30 | 1 PE | 17 | 13 | 5 including 1 ectopic | 8 | 2 | 5 | 5 | 12 |
| Bangerter et al | 9 | 17 | 1 TIA, 2 acquired vWD, 3 vaginal bleeds, 2 epistaxis | 11 | 6 | 6 | 0 | 0 | 0 | 3 | 8 |
| Niittyvuopio et al | 16 | 40 | 1 eclampsia, 2 PET, 1 vaginal bleed | 26 (1 twin) | 15 | 13 | 2 | 1 | 0 | 2 | 23 |
| Gangat et al | 36 | 63 | 1 PET, 2 hematoma, 1 PPH | 38 | 25 | 22 including 1 ectopic, 1 TOP | 3 | Detail not available | 1 | 1 | 37 |
| Melillo et al | 92 | 122 | 5 DVT, 3 PET, 1 PPH | 92 | 30 | 23 | 7 | 2 | 1 | 12 | 80 |
| Passamonti et al | 78 | 113 | 5 PET | 44 | 34 | Detail not available | Detail not available | 7 | Detail not available | Detail not available | Detail not available |
| Palandri et al | 13 | 24 | Detail not available | 15 | 9 | Detail not available | Detail not available | 0 | 1 | 0 | Detail not available |
| Total | 295 | 470 | — | 284/470 | 152/470 | 83/333 | 26/333 | 14/386 | 9/357 | 33/357 | 191/333 |
| (60%) | (32%) | (25%) | (8%) | (4%) | (3%) | (9%) | (57%) |
| Author Ref. | Patients, n | Pregnancies, n | Treatment During Pregnancy | High Risk | Maternal Outcome | Live Births, n | Pregnancy Loss, n | First Trimester Miscarriage | Stillbirth (Gestation) | IUGR | Placental Abruption | Live Birth <37/40 | Live Birth FTD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crowley et al | 1 | 1 | Aspirin + dipyridamole | No | Death | 0 | 1 | 1 TOP | 0 | 0 | 0 | 0 | 0 |
| Centrone et al | 1 | 3 | Nil | No | Alive | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 1 |
| Ferguson et al | 1 | 2 | Nil | No | Alive, PET | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 PET |
| Ruch and Klein | 1 | 2 | None | No | Alive, PET | 1 | 1 | 0 | 1 (35/40) PET | 1 | 0 | 0 | 1 |
| Subtil et al | 1 | 3 | Aspirin, heparin, venesection | No | Alive, PE postpartum | 1 | 2 | 0 | 2 (24/40 and 28/40) | 2 | 0 | 1 (32/40) | 0 |
| Hochman and Stein | 1 | 4 | Nil | Yes | Alive, PET | 2 | 2 | 0 | 2 (5 & 7 months) PET | 0 | 0 | 1 (7 months, PET), 1 (8 months) | 0 |
| Harris and Conrad | 2 | 2 | Nil | No | Alive, PPH | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Ruggeri et al | 1 | 2 | Heparin 3/52 postpartum | No | Alive, PE 24/7 postpartum | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| Pata et al | 1 | 1 | Hydroxyurea 9/40 then nil | No | Alive | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Robinson et al | 8 | 18 (1 twin) | Varied: venesection, aspirin, interferon LMWH, vitamin C+E | No | Alive, 1 PET | 11 | 7 | 4 | 2 | 3 | 0 | 1 (34/40, IUGR), 1 (36/40), 1 (26/40) (NND) | 9 |
| Total | 18 | 38 | — | 1 yes | 1 death, 4 PET, 2 PE, 1 PPH | 22, 3 neonatal deaths | 16 | 8 | 7 | 6 | 0 | 6 | 17 |
| Author Ref. | Patients, n | Pregnancy, n | Treatment Prepregnancy | Treatment During Pregnancy | Maternal Outcome | First Trimester Miscarriage | Stillbirth (Gestation) | IUGR | Placental Abruption | Live Birth Premature Delivery <37 wk |
|---|---|---|---|---|---|---|---|---|---|---|
| Taylor et al | 1 | 1 | Supportive | Supportive | No complications | 0 | 0 | 0 | 0 | 1 elective induction at 36 wk |
| Gotic et al | 1 | 1 | None | None | No complications | 0 | 30 (placental infarctions) | 0 | 0 | 0 |
| 2 | None | None | No complications | 0 | 27 (placental infarctions) | 0 | 0 | 0 | ||
| 3 | Interferon-α | Interferon-α | No complications | 0 | 0 | 1 | 0 | 1 elective delivery at 34 wk due to IUGR, birth weight 2000 g | ||
| Tulpule | A | 3 (2 preceding diagnosis PMF) | Aspirin | Aspirin | Disseminated TB | 0 | 0 | 0 | 0 | 1 FTND |
| B | 1 | Aspirin | Aspirin, LMWH | Postpartum hemorrhage | 0 | 0 | 0 | 0 | 1 FTND | |
| B | 2 | Aspirin | Aspirin, LMWH | No complications | 0 | 24/40 cardiac malformation | 0 | 0 | 0 | |
| B | 3 | Aspirin | Aspirin, LMWH | No complications | 1 | 0 | 0 | 0 | 0 | |
| Total | 4 | 8 | 3 | 3 | 0 | 3 | 1 | 0 | 4 |
Pregnancy outcome data and risk factors for MPN
ET is the commonest MPN in women of childbearing age, and a significant number of cases of pregnancy have been reported in the literature, but these data are insufficient to devise evidence-based management guidelines. A meta-analysis reported the outcome of 461 pregnancies in women diagnosed with ET. The live birth rate was 50% to 70%; first trimester loss occurred in 25% to 40% and late pregnancy losses in 10%. Rates of placental abruption (3.6%) and intrauterine growth restriction (IUGR) (4.5%) were higher than in the general population. Postpartum thrombotic episodes were reported in 5.2% of pregnancies and pre-/postpartum hemorrhage in 5.2%. A summary of 208 historical cases of ET collated from case series that included greater than 6 pregnancies produced comparable data ( Table 1 ). The literature for pregnancies affected by PV is sparse; pregnancy outcome in the authors’ own case series of 18 pregnancies in PV combined with 20 historical reports was concordant with the pregnancy outcomes in ET, and is summarized in Table 2 . In PV first trimester loss was the most frequent complication (21%), followed by late pregnancy loss (18%), IUGR (15%), and premature delivery (13%), which included 3 neonatal deaths resulting in a 50% survival rate. Maternal morbidity was also significant including 3 thromboses, 1 large postpartum hemorrhage, 4 cases of preeclampsia, and 1 maternal death associated with evidence of a deep vein thrombosis, pulmonary emboli, sagittal sinus thrombosis, and disseminated intravascular coagulation. The literature regarding PMF is more limited. A report of 4 pregnancies in PMF combined with 4 historical cases suggested a 50% risk of fetal loss; no maternal complications of thrombosis or disease progression were noted, but the numbers are probably too small to draw any firm conclusions ( Table 3 ).
| Author Ref. | Patients, n | Pregnancies, n | Maternal Outcome | Live Birth Total | Pregnancy Loss Total | Loss <12/40 | Loss >12/40 | IUGR | Placental Abruption | Live Birth Premature Delivery <37/40 | Live Birth FTD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Belluci et al | 3 | 11 | Detail not available | 4 | 7 | 6 | 1 | Detail not available | 1 | 2 | 2 |
| Beard et al | 6 | 9 | 1 phlebitis, 1 leg ulcer, 1 PPH | 8 | 1 | 1 | 0 | 0 | 0 | 1 | 7 |
| Leone et al | 8 | 10 | Detail not available | 7 | 3 | 0 | 3 | Detail not available | 0 | 0 | 7 |
| Pagliaro et al | 9 | 15 | 2 VTE, 2 TIA, 1 hemorrhage | 9 | 6 | 4 including 1 TOP | 2 | 2 | 0 | 4 | 5 |
| Randi et al | 13 | 16 | 3 VTE | 13 | 3 | 3 | 0 | 0 | 0 | 3 | 10 |
| Cincotta et al | 12 | 30 | 1 PE | 17 | 13 | 5 including 1 ectopic | 8 | 2 | 5 | 5 | 12 |
| Bangerter et al | 9 | 17 | 1 TIA, 2 acquired vWD, 3 vaginal bleeds, 2 epistaxis | 11 | 6 | 6 | 0 | 0 | 0 | 3 | 8 |
| Niittyvuopio et al | 16 | 40 | 1 eclampsia, 2 PET, 1 vaginal bleed | 26 (1 twin) | 15 | 13 | 2 | 1 | 0 | 2 | 23 |
| Gangat et al | 36 | 63 | 1 PET, 2 hematoma, 1 PPH | 38 | 25 | 22 including 1 ectopic, 1 TOP | 3 | Detail not available | 1 | 1 | 37 |
| Melillo et al | 92 | 122 | 5 DVT, 3 PET, 1 PPH | 92 | 30 | 23 | 7 | 2 | 1 | 12 | 80 |
| Passamonti et al | 78 | 113 | 5 PET | 44 | 34 | Detail not available | Detail not available | 7 | Detail not available | Detail not available | Detail not available |
| Palandri et al | 13 | 24 | Detail not available | 15 | 9 | Detail not available | Detail not available | 0 | 1 | 0 | Detail not available |
| Total | 295 | 470 | — | 284/470 | 152/470 | 83/333 | 26/333 | 14/386 | 9/357 | 33/357 | 191/333 |
| (60%) | (32%) | (25%) | (8%) | (4%) | (3%) | (9%) | (57%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



