Anemia
INTRODUCTION
Anemia is defined as a decrease in the red blood cell mass circulating in the bloodstream, and derives from an imbalance in the production and loss of erythrocytes. Symptoms and signs associated with anemia result from impaired oxygen delivery to the tissues. Common symptoms include fatigue, malaise, weakness, dyspnea on exertion, palpitations, and chest pressure. Patients may manifest additional overt signs such as pallor, tachycardia, impaired mentation, high-output congestive heart failure, shock, and death. The 1968 World Health Organization (WHO) criteria define anemia as hemoglobin less than 12 g/dl in women and hemoglobin less than 13 g/dl in men. The current working definition of anemia is a hemoglobin level that is two standard deviations below the mean hemoglobin level for a given sex and age.
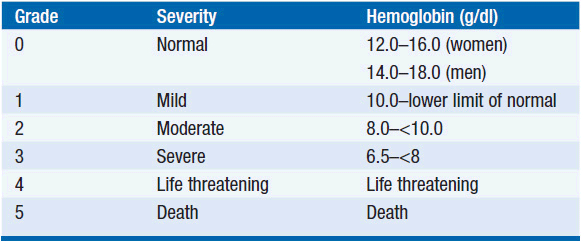
Among patients with cancer, anemia is a prevalent complication of both the disease and its treatment. Nearly 50% of patients have laboratory evidence of anemia at the time of diagnosis with cancer, although it may be initially quite subtle and insidious in onset. With hematologic malignancies, anemia is coincident in as many as 70% of patients. Cancer patients with a particularly increased risk for anemia are those with a low hemoglobin before the diagnosis of cancer, those with lung or gynecologic cancers, and those receiving platinum-based therapy, and female sex (1). Due to the prevalence of anemia with cytotoxic chemotherapy, grading systems have been established to standardize reporting of myelosuppression in clinical studies and to guide clinical decision-making. The grading system offered by the National Cancer Institute is presented in Table 18-1 (2).
Anemia has been shown to decrease quality of life in cancer patients (3). The correlation between fatigue and hemoglobin level is particularly strong, establishing fatigue as a modifiable risk factor for clinical trials of transfusion or erythropoietins (EPOs). A negative impact of anemia on cancer patient prognosis and survival has been reported in both solid and hematologic malignancies (4). Because anemia in the cancer patient is frequently multifactorial, the appropriate diagnostic evaluation and therapeutic interventions must be individualized to fit the cause, the severity of anemia, and the clinical setting. The mainstay of treatment is treating the underlying cause or supportive care with packed red blood cell transfusions and EPO with or without iron supplementation.
ERYTHROPOIESIS
Hematopoiesis and the size of each compartment within its developmental hierarchy, including red blood cell production, are tightly controlled by a dynamic balance of hematopoietic stem cell (HSC) self-renewal and differentiation through subsequent compartments to mature effector cells. HSCs give rise to the common myeloid progenitor (CMP) and subsequently megakaryocyte/erythroid progenitor (MEP) in response to growth factor and cytokine stimulation, as well as instruction by the bone marrow niche. MEP eventually gives rise to red blood cells under the influence of EPO hormone. After nuclear extrusion in the marrow, immature red cells called reticulocytes are released into the circulation. The reticulocytes retain some ribosomes and mRNA that are generally destroyed after the first day in the circulation. The resulting cell is a mature red blood cell. The marrow produces more than a million erythrocytes per second, compensating for the normal 1% daily loss. EPO, a glycoprotein hormone secreted by the kidney (and to a lesser extent by the liver) in response to hypoxia, is primarily responsible for the pace of red cell production, provided that the HSC is normal and adequate supplies of iron are available.
Dietary iron is absorbed in the duodenum and proximal jejunum by apical transporters on enterocytes. The recommended daily allowance (RDA) of iron for adults is 18 mg/day. Absorbed iron then passes across the gut basement membrane into the circulation where it binds transferrin and enters the liver, the primary storage site. Particularly relevant in patients with cancer and systemic inflammatory diseases is the production of hepcidin by the liver and other cells, which impairs iron reutilization by increasing duodenal crypt cell and macrophage iron retention and downregulating iron transporters (5). Elevated hepcidin levels in patients with cancer may impair erythropoiesis. Transferrin receptors on erythrocytic precursors mediate iron uptake.
Red cell production, like other processes that require DNA synthesis, also requires adequate vitamin B12 and folate. Dietary folate derives from leafy vegetables and animal products. The RDA of folate for adults is 50 mg/day. Dietary folate, mainly in the form of 5-methyltetrahydrofolate, is absorbed in the jejunum, exhibits significant enterohepatic recirculation, and ultimately enters HSCs by the reduced folate receptor. It is stored in the liver and other tissues as a polyglutamated derivative, and released as needed into the circulation. Dietary cobalamin is derived from animal products. The RDA of cobalamin is 2 μg/day. The first step in cobalamin absorption requires splitting the dietary vitamin from binding proteins in food through the action of acid and pepsin in the stomach. This step is followed by additional proteolysis by pancreatic enzymes, binding of the free cobalamin to intrinsic factor (a glycoprotein secreted by the stomach), and receptor-mediated internalization in the ileum. Medications that impair gastric acid secretion and atrophic gastritis can impair the essential process of splitting vitamin B12 from food binders and interfere with intrinsic factor production.
Under normal circumstances, an erythrocyte circulates for 120 days. Thereafter, red cells are removed from circulation by tissue macrophages of the reticuloendothelial system (RES). Heme-bound iron is recycled and stored as ferritin and hemosiderin in the liver, spleen, and bone marrow. Iron stores may be mobilized by release into the plasma and oxidation by ceruloplasmin. Important additional tissues contribute to red cell homeostasis, such as endothelial and serum control of hemostasis and cardiorenal maintenance of plasma volume.
DIAGNOSTIC EVALUATION
The diagnostic evaluation of anemia aims to identify etiologies upon which treatment can be based. A detailed history provides important insights into the pace of development of anemia, and informs the interpretation of laboratory studies. The evaluation of anemia requires a detailed family history, as well as consideration of the family’s ethnic, racial, and geographic origins, which may suggest parasites, sickle cell inheritance or thalassemia, or pernicious anemia as causes of anemia. As patients with cancer are frequently treated with agents that cause oxidative stress, glucose-6-phosphate dehydrogenase deficiency with drug-induced hemolysis may become a relevant diagnostic consideration, particularly in patients of Mediterranean and African American origin.
Laboratory evaluation serves to quantify the degree of anemia and the immediate risk posed by the red cell deficit. The hemoglobin concentration in whole blood is routinely used to define anemia. The physiologic reserve of the patient may play a dominant role in the level of symptoms associated with a particular hemoglobin level. The time frame over which the anemia developed and the presence of concurrent illness all influence the degree of symptoms for a particular level of hemoglobin.
Laboratory evaluation of peripheral blood and, if necessary, bone marrow usually yields the cause of anemia. A careful review of the blood smear may reveal morphologic clues useful in confirming the underlying etiology (see below). A complete blood count with differential is obtained to determine if additional hematopoietic lineages are affected. Measurement of the serum creatinine is used to rule out renal failure as a contributing cause or complication. The red cell indices, such as mean corpuscular volume and mean corpuscular hemoglobin, differentiate microcytic anemia from megaloblastic anemia. Among microcytic anemias, the red cell distribution width (RDW) distinguishes between iron deficiency (wide RDW) and thalassemia (narrow RDW). Iron studies (iron, ferritin, and total iron binding capacity) are useful to differentiate iron-deficiency anemia from the anemia of chronic disease. Examination of the stool for occult blood is essential to rule out chronic gastrointestinal bleeding.
Additional studies may diagnose specific etiologies. Reticulocytosis, elevation of serum lactate dehydrogenase and indirect bilirubin, and depressed serum haptoglobin suggest hemolysis. Serum free hemoglobin or urinary hemosiderin reflects intravascular hemolysis. A positive direct antiglobulin (Coombs) test confirms autoimmune hemolytic anemia. In patients with macrocytic anemia, measurement of red blood cell folate levels and plasma homocysteine indicate the presence of folate deficiency, while serum methylmalonic acid and cobalamin are measured to establish B12-deficiency anemias. Low-serum levels of thyroxine or testosterone may also contribute to anemia. In patients with multilineage cytopenias, refractory anemia, or malignancies commonly metastatic to bone, a bone marrow aspirate and biopsy and cytogenetics may establish tumor replacement (myelophthisis) or treatment-related myelodysplasia as the cause.
CLASSIFICATION OF ANEMIA IN PATIENTS WITH CANCER
Anemia can be classified as either relative or absolute. Relative anemia occurs with increases in plasma cell volume, such as with volume overload or pregnancy. Absolute anemia reflects a true decrease in the red cell mass. Causes of anemia in the cancer patient may be ascribed to three fundamental processes:
 DECREASED RED CELL PRODUCTION (THE DOMINANT FACTOR IN ANEMIA IN CANCER PATIENTS)
DECREASED RED CELL PRODUCTION (THE DOMINANT FACTOR IN ANEMIA IN CANCER PATIENTS)
• Myelosuppression due to chemotherapy or radiation therapy is the most common etiology of anemia in the cancer patient. Multiagent, dose-intense or dose-dense regimens of nonselective cytotoxins are the most likely causes. Regimens employing cisplatin, taxanes, or alkylating agents are often implicated. Cycles of common regimens lead to progressive anemia, with incomplete recovery between cycles. The anemia is typically normocytic or macrocytic with a low-reticulocyte index.
• Replacement of the normal bone marrow elements by malignancies such as lymphoma, multiple myeloma, or leukemia and less commonly by solid tumors such as metastatic prostate or breast cancer may lead to anemia. In such patients, progressive, normocytic anemia is accompanied by a low-reticulocyte index. Rarely, in such cases, the peripheral blood smear contains early precursors of both the myeloid and erythroid lineages (a leukoerythroblastic response).
• Abnormal stem cell function or impairment in maturation can cause an anemia of underproduction of red cells, as with aplastic anemia, pure red cell aplasia, myelodysplastic syndrome, or acute leukemia. Myelodysplasia is an infrequent sequel of chronic alkylating agent therapy or combined modality therapy with radiation therapy and chemotherapy. A macrocytic anemia with a low-reticulocyte index may be present. The bone marrow examination typically demonstrates dysplastic, immature myeloid, and erythroid forms, and abnormal cytogenetics.
• Due to compromised nutritional status, malabsorption, treatment-related anorexia, and the hypermetabolic demands of the neoplastic process, cancer patients may manifest folate and/or vitamin B12-deficiency anemia. For example, there are reports of cases of pernicious anemia in gastric cancer patients. In the absence of concurrent iron deficiency, both folate and vitamin B12 deficiency will result in a macrocytic or megaloblastic anemia with depressed reticulocyte index and elevated plasma homocysteine. Iron-deficiency anemia is prevalent in up to 30%–60% of cancer patients and includes functional iron deficiency, which may result from blood loss or prolonged EPO use, requiring oral or intravenous supplementation. RCTs have shown superior efficacy of iv iron supplementation over oral iron or no iron supplementation in reducing blood transfusion requirement, raising hemoglobin level, and improving quality of life in anemic cancer patients treated with an erythrocyte stimulating agent (ESA) (6).
• Reduced endogenous EPO levels are reported in cancer patients with anemia in the absence of other obvious causes (7). A normocytic anemia with low reticulocytes is seen on the peripheral smear. Renal impairment may contribute to decreased EPO production as a consequence of the malignancy (i.e., multiple myeloma) or therapy (i.e., cisplatin). Serum EPO level may confirm the diagnosis, but is rarely needed to guide the therapeutic intervention.
• Inflammatory cytokines, such as tumor necrosis factor alpha, interleukin-1, interleukin-6, and interferon gamma, may be increased in cancer patients as a consequence of tissue destruction, inflammation, or tumor secretion, and may suppress erythropoiesis by inhibiting survival and differentiation of erythroid progenitor cells.
 INCREASED RED CELL DESTRUCTION (RARE IN CANCER PATIENTS)
INCREASED RED CELL DESTRUCTION (RARE IN CANCER PATIENTS)
• Autoimmune hemolytic anemia is observed occasionally in B-cell lymphoproliferative disorders, such as chronic lymphocytic leukemia and non-Hodgkin lymphoma. Fludarabine or allogeneic stem cell transplantation may unveil or exacerbate autoimmune anemias. A Coombs test will usually identify a warm agglutinin; other findings are an elevated LDH and depressed haptoglobin. Brisk hemolysis may result in an elevated indirect bilirubin and critically low hemoglobin. The extent of marrow involvement and timing of myelosuppressive therapy will affect the degree of reticulocytosis. Here, effective treatment should include supportive measures, immunosuppressive therapy (i.e., glucocorticoids, intravenous immunoglobulin, or rituximab), and agents targeting the underlying disease.
• Microangiopathic hemolytic anemia occasionally accompanies gastrointestinal malignancies, immunosuppression with cyclosporine or tacrolimus, or exposure to chemotherapeutic agents such as gemcitabine and mitomycin C. Chronic disseminated intravascular coagulopathy can manifest as a mild to moderate anemia.
• Hypersplenism, characterized by increased red cell destruction in the absence of a positive Coombs test, may accompany hematologic malignancies, especially lymphomas. Portal hypertension may lead to a normocytic anemia due to splenic pooling. Patients with significant, refractory hypersplenism may respond well to splenectomy.
 BLOOD LOSS
BLOOD LOSS
• Acute and chronic blood loss is a frequently presenting problem in patients with gastrointestinal and genitourinary malignancies. Disease or chemotherapy-related thrombocytopenia may contribute to the risk of bleeding. Brisk hemorrhage due to tumor erosion into medium and large blood vessels may be life threatening, as observed in locally advanced head and neck or pancreatic cancer. Acute intratumoral hemorrhage is uncommon, but described in hepatocellular carcinoma, sarcoma, metastatic melanoma, and other rapidly proliferative, bulky solid tumors. Unusual causes of life-threatening tumor hemorrhage include bleeding from cavernous hemangioma, splenic hemangiosarcoma, and tumors metastatic to the ovary. Bevacizumab is associated with hemorrhage occasionally, particularly in patients with lung cancer.
• Chronic hemorrhage is a frequent complication of gastrointestinal tumors and gynecologic tumors, in particular endometrial cancer. Often, blood loss is surreptitious and manifests as an iron-deficiency anemia.
INTERVENTION
The adverse consequences of cancer-related anemia warrant intervention appropriate to symptoms and comorbidities. The variable causes of anemia in the cancer patient require specific, directed therapies. Rapid identification of the cause(s) of anemia should prompt the appropriate intervention to correct causes of blood loss, hemolysis, clotting defects, or other reversible processes. Most often, however, no specific and reversible cause is identified other than therapy and the anemia of chronic illness. To replenish red cell mass, two interventions are most often considered: red blood cell transfusion and recombinant EPO with or without iron supplementation.
Transfusion of red blood cells acutely alleviates the symptoms of anemia. However, transfusion is not without risk. Although extremely rare, complications of blood transfusion include volume overload, infection (bacterial, HIV, CMV, HBV, HCV, HTLV), acute transfusion reactions, iron overload, transfusion-related acute lung injury, and allo-immunization. Unless the donor cells are irradiated, there is a risk that donor lymphocytes could induce fatal graft-vs-host disease. Nonetheless, with acute or severe anemia, transfusion may prove life-saving. Under less emergent conditions, the decision to restore red cell mass through transfusion or the use of EPO requires clinical judgment. Evidence-based guidelines for transfusion have been published by a number of organizations, and their conclusions are consistent with a large study that compared restrictive versus liberal transfusion in the critically ill (8). For reasons that remain largely undefined, efforts to keep hemoglobin levels near normal in patients with acute illness are actually associated with poorer overall survival. Without evidence of severe cardiac disease, it is appropriate to restrict transfusion to those patients with a hemoglobin less than 7 g/dl, maintaining levels between 7 and 9 g/dl. The rate of transfusion depends on the severity of symptoms related to hypoxia (dyspnea, fatigue, changes in mental status, tachycardia, angina), taking care to avoid acute fluid overload. A recent Cochrane review analyzed the benefit of blood transfusions in patients with advanced cancer. Although there were no randomized controlled trials (RTCs) available at the time of analysis, meta-analysis of available data pooled from 12 different studies showed a subjective response rate of 31%–70% posttransfusion with effects waning by day 14. However, there was a high 14-day mortality associated with transfusions, and additional studies will be required to shed light on causality versus inappropriate use of transfusions in dying patients (9).
Initially marketed for anemia in advanced renal disease, recombinant EPO was approved by the US FDA for the treatment of cancer-related anemia in 1993 based on quality-of-life endpoints. The two commercially available preparations of recombinant EPO in the United States are epoetin alpha (Epogen, Amgen; Procrit, Ortho Biotech) and the second-generation erythrocyte stimulating agent (ESA) with a longer half-life, darbepoetin alpha (Aranesp, Amgen). Recombinant epoetin alpha is almost identical to the endogenous human glycoprotein, EPO, differing importantly in glycosylation of key residues that prolong half-life. Darbepoetin alpha differs from endogenous EPO at five amino acid residues, allowing for hypoglycosylation and an even more prolonged serum half-life.
Modest clinical efficacy of epoetin alpha and darbepoetin alpha has been established in pivotal, placebo-controlled, registration studies of patients with solid and hematologic malignancies (10–12). Epoetin alpha and darbepoetin alpha demonstrate comparable efficacy in cancer-related anemia. Epoetin alpha was initially studied as a three-time weekly formulation in FDA registration studies. Subsequent clinical trials have established the safety and activity of weekly, high-dose treatment. Weekly epoetin alpha (40,000 units, adjusted in subsequent doses according to the increase in hemoglobin) and darbepoetin alpha every 2 weeks (200 mcg, adjusted) are equally effective (13).
In these and subsequent studies, a consistent, small positive effect has been observed in hemoglobin levels, diminished transfusion requirement and quality of life, though the response develops slowly over several weeks. Evidence-based guidelines have been established and, in general, a consensus has been reached that EPO can produce a symptomatic benefit, but various guideline-developing groups differ in their target hemoglobin. Quality-of-life data support an upper boundary hemoglobin of 12 g/dl (14). However, data have begun to raise the question of whether EPO might protect tumors against therapeutic interventions like radiation therapy and chemotherapy.
An increased risk of adverse complications such as hypertension, venous thromboembolism, and cardiovascular events is reported with recombinant EPO. An international placebo-controlled study of epoetin alpha in patients with metastatic breast cancer was terminated early due to an unexpected, increased recurrence rate and mortality in the treatment arm (15). A study of epoetin beta (NeoRecormon, Roche), approved in Europe for cancer-related anemia, illustrated increased thrombotic and cardiovascular events among treated patients (16). At least eight clinical studies to date have generated data suggesting that EPO is associated with increased risk of cancer recurrence, cancer progression, or death (reviewed in [17]).
For example, the Danish Head and Neck Cancer Study Group trial (DAHANCA 10) compared radiotherapy-to-radiotherapy plus darbepoetin alpha (target hemoglobin 14.0–15.5 g/dl) in the treatment of advanced head and neck cancer. Three-year locoregional control and overall survival were both worse in patients treated with darbepoetin alpha (http://conman.au.dk/dahanca). A double-blind study of 989 patients treated with darbepoetin alpha (target hemoglobin 12 g/dl) or placebo failed to demonstrate a favorable effect of darbepoetin on red cell transfusion requirements. However, patients treated with darbepoetin demonstrated an increase in mortality. A similar study of weekly epoetin alpha (40,000 IU; target hemoglobin 12–14 g/dl) in anemic patients with lung cancer was closed prematurely due to increased mortality in treated patients. Median time to death was 68 days with ESA versus 131 days with placebo. Accordingly the FDA has issued a boxed warning for EPO products (http://www.fda.gov/cder/drug/infopage/RHE/default.htm).
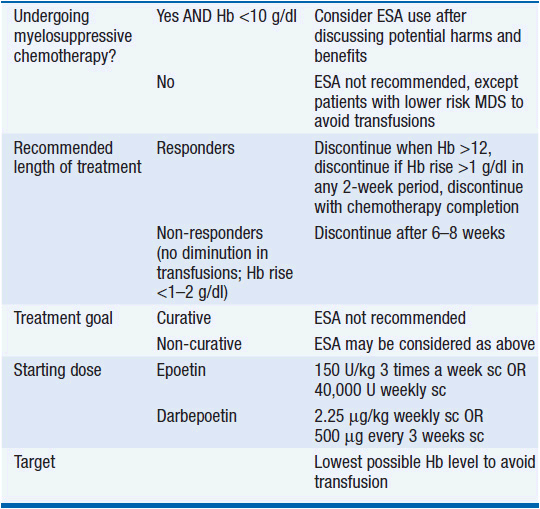
In the most recent Cochrane review of 91 RCTs on managing anemia in cancer patients using ESA, the use of ESAs significantly reduced the need for red blood cell transfusions (RR 0.65), and was associated with a better hematologic response, as well as suggestion of improved quality of life. However, there was strong evidence that ESAs increase mortality (HR 1.17), and less strong evidence that they decrease overall survival (HR 1.05). There was a significantly increased risk of thromboembolic complications (RR 1.52), as well as hypertension (RR 1.30) and inconclusive evidence regarding tumor response (18). As a result of these studies, the American Society of Hematology and the American Society of Clinical Oncology published practice guidelines on the use of ESAs in adult patients with cancer (19), which are outlined in Table 18-2. In summary, EPOs are not FDA approved for use in cancer patients not receiving myelosuppressive chemotherapy with the exception of patients with low-risk myelodysplastic syndromes (MDS) to avoid transfusions, and they are not recommended for patients with hemoglobin >10. EPO is being evaluated clinically for its capacity to limit the size of strokes and myocardial infarcts through its action to protection of hypoxic cells from cell death. It is possible that this protective effect on dying cells will be beneficial in some settings, but all evidence thus far suggests that it is detrimental in patients with cancer. Until the issue is better defined, the use of EPO might wisely be confined to those settings where it is a component of palliative care. Its use as a component of curative regimens is undefined and potentially harmful.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree