ANDROGENS: PAST AND PRESENT
The popular belief that failure of testicular function was responsible for symptoms of old age in men stimulated early attempts to isolate an active testicular substance that would rejuvenate aging men. In 1889, Brown-Sequard reported that an extract he prepared from dog and guinea pig testes and administered to himself resulted in increased vigor, strength, intellectual capacity, and sexual potency.1 Because of his stature in the scientific community, many people in the public and medical profession began using testicular extracts, but others castigated Brown-Sequard. Although his aqueous extract probably was devoid of steroid hormone and bioactivity, the controversy concerning his “elixir of life” stimulated further studies of internal secretions of glands, from which evolved the science of modern endocrinology.
Soon after the discovery of androgenic substances in the urine, urinary extracts were reported to increase the frequency. Because of their limited availability and weak activity, however, urinary androgens were not widely used. In the mid-1930s, testosterone was isolated and identified as the major androgenic principle of the testes. Soon thereafter, the chemical structure of testosterone was elucidated, and the hormone was synthesized and came to be used clinically in the treatment of male hypogonadal states. Because of the short duration of action of testosterone, numerous analogs and derivatives of testosterone with greatly prolonged action were developed.
Today, the principal clinical use of androgens remains the treatment of androgen deficiency resulting from hypogonadism and delayed puberty.2,3 and 4 Androgens are also used to stimulate erythropoiesis in hypoproliferative anemias related to renal or bone marrow failure; to treat micropenis and microphallus (see Chap. 93); to treat hereditary angioneurotic edema; as adjuvant hormonal therapy for female breast cancer (see Chap. 224); topically to treat vulvar lichen sclerosus; and to stimulate bone formation in the treatment of osteoporosis.2,3 and 4
Preliminary studies have suggested a number of potential uses of androgens for treatment of clinical syndromes associated with wasting syndromes (e.g., acquired immunodeficiency syndrome [AIDS] and cancer); chronic illnesses, such as renal failure and chronic obstructive pulmonary disease (COPD); long-term use of certain medications (e.g., glucocorticoid therapy); autoimmune rheumatologic diseases (e.g., rheumatoid arthritis); and aging. Evidence is also emerging that androgens may be useful in treating reduced libido and in increasing bone mass in postmenopausal women. Finally, interest is renewed in investigating the use of androgens to promote protein anabolism in catabolic states (e.g., after major trauma or surgery, burns, space travel, and spinal cord injury). Although the short- and long-term benefits and risks of androgen therapy in these conditions are not fully established, the potential for expanded clinical uses of androgens has stimulated interest in the development of new androgens and alternative androgen formulations, and has once again sparked both public and physician enthusiasm for the use of testosterone as an agent to prevent frailty in aging men.
Although androgens have been used to treat children with short stature, their value for this purpose is dubious, and such a use is inappropriate. Extremely high dosages of androgenic, anabolic steroids are increasingly consumed by athletes to improve performance and by adolescents to enhance appearance. Whether androgens improve athletic performance is uncertain, and the high dosages are often associated with severe side effects. Despite condemnation by the medical community and by athletic organizations, this form of androgen abuse remains widespread. The potential for androgen abuse has resulted in reclassification of androgen and androgenic anabolic steroid preparations as Schedule III controlled substances by the U.S. Food and Drug Administration (FDA). With the enactment of the Dietary Supplement Health and Education Act of 1994, however, preparations containing the androgens dehydroepiandrosterone (DHEA) and Δ4-androstenedione (androstenedione), both of which may be converted to testosterone in the body, became available over the counter as “dietary supplements” free of FDA regulation. Despite the lack of evidence for their benefits or risks, DHEA and androstenedione are being abused by athletes to enhance strength and performance, and by men and women in the hope of “preventing the aging process.”
Antiandrogens are compounds that bind to the androgen receptor and competitively inhibit androgen binding, thereby antagonizing androgen action at target organs. Clinically, they are useful in the treatment of androgen-dependent malignancies (e.g., prostate and male breast cancer) and conditions such as hirsutism and acne (see Chap. 101 and Chap. 225).
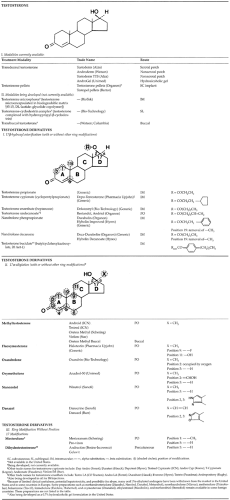
PHARMACOLOGY OF ANDROGEN PREPARATIONS
Because of the short duration of testosterone action, pharmacologic strategies have been developed to achieve more sustained
blood levels of testosterone and thereby prolong its androgenic action. The development of novel methods of testosterone administration and chemically modified analogs of the native testosterone molecule are the two major pharmacologic strategies used to prolong androgen action.2 In general, the chemical modifications of testosterone involve esterification of the 17β-hydroxyl group or alkylation of the 17α-position of the D ring, with or without other modifications of the ring structure of native testosterone (Table 119-1). Mesterolone and dihydrotestosterone (DHT) are androgen preparations that have A-ring modifications without any alterations at the 17-position of the D ring (see Table 119-1).
blood levels of testosterone and thereby prolong its androgenic action. The development of novel methods of testosterone administration and chemically modified analogs of the native testosterone molecule are the two major pharmacologic strategies used to prolong androgen action.2 In general, the chemical modifications of testosterone involve esterification of the 17β-hydroxyl group or alkylation of the 17α-position of the D ring, with or without other modifications of the ring structure of native testosterone (Table 119-1). Mesterolone and dihydrotestosterone (DHT) are androgen preparations that have A-ring modifications without any alterations at the 17-position of the D ring (see Table 119-1).
TESTOSTERONE
Orally administered testosterone is rapidly absorbed from the gastrointestinal tract into the portal blood and accumulated in the liver. Because it is efficiently degraded by the liver, very little testosterone reaches the systemic circulation, and sustained blood levels are very difficult to maintain. No oral forms of unmodified testosterone are available in the United States. A microparticulate form of testosterone has been administered to a small number of hypogonadal men in Europe and has been shown to achieve therapeutic blood levels of testosterone in some patients.5 Absorption of this preparation is erratic, however, and very large dosages (200–400 mg per day) taken several times a day are required to maintain adequate serum testosterone levels. Furthermore, the long-term toxicity to the liver of this large burden of testosterone is unknown. Intramuscular injections of aqueous native testosterone are also rapidly absorbed from the injection site and promptly degraded by the liver. Therefore, unmodified testosterone administered either orally or parenterally is impractical for achieving sustained physiologic testosterone levels in blood and androgenic effects on target organs.
TRANSDERMAL TESTOSTERONE DELIVERY
Currently, three transdermal testosterone patches are approved for use in androgen replacement therapy for hypogonadal men (see Table 119-1): a scrotal matrix patch (Testoderm), the first to be marketed; a nonscrotal, permeation-enhanced, reservoir patch (Androderm or Andropatch [United Kingdom]); and a nonscrotal reservoir patch that does not contain permeation enhancers (Testoderm TTS).6 Because a normal adult man produces ˜7 mg per day of testosterone (i.e., up to 100-fold the daily production of estradiol), testosterone patches are larger and require more frequent (daily) application than do estrogen patches.
The scrotal Testoderm patch consists of an ethylene-vinyl acetate copolymer containing testosterone within this matrix.7,8 When applied to scrotal skin, the 40 cm2 or 60 cm2 patches deliver 4 or 6 mg of testosterone over 24 hours, respectively. The unusual superficial vascularity of scrotal skin permits 5- to 40-fold greater testosterone absorption from this site than from other skin sites. Because of initial problems with adhesion of these patches to scrotal skin, thin, lightly adhesive strips were incorporated onto the patch. Daily application in the morning on the scrotum of hypogonadal men produces physiologic levels of testosterone that mimic the normal circadian rhythm of testosterone in young men, with peak testosterone levels reached within 2 to 4 hours. Because these patches do not contain permeation enhancers, they are generally well tolerated, with only occasional itching (˜7%)and moderate skin irritation (˜5%). The use of this patch, however, does require an adequate-size scrotum that is clean, dry, and shaven for optimal adhesion and effectiveness; this limits its acceptability and clinical utility. Also, supraphysiologic concentrations of DHT are produced, as a result of the high 5α-reductase activity in scrotal skin. The physiologic significance of high circulating DHT levels on androgen-responsive target organs (e.g., prostate) is not known, but careful monitoring for adverse androgenic effects is recommended.
The nonscrotal Androderm transdermal system consists of an adhesive patch with a central reservoir containing testosterone and permeation enhancers in an alcohol-based gel that are delivered to skin through a microporous polyethylene membrane.9,10 and 11 Permeation enhancers facilitate testosterone absorption through nonscrotal sites (e.g., back, abdomen, thighs, or upper arm). In hypogonadal men, nightly application of a single 5 mg (44 cm2) or two 2.5 mg (37 cm2) patches on nonscrotal skin also maintains serum testosterone concentrations in the normal physiologic range with a circadian rhythm similar to that of young men. The Androderm patch causes more local skin irritation (˜30% of users) than do scrotal Testoderm patches (˜5%).10 Unlike scrotal patches, they may cause allergic contact dermatitis (˜12%) and, uncommonly, a burn-like blister reaction, which is usually associated with placement of the patch over bony prominences. The severity and incidence of skin irritation is reduced by coapplication of triamcinolone acetonide (0.1%) cream under the drug reservoir. However, skin irritation limits the acceptability and use of this nonscrotal patch. Unlike with the scrotal testosterone patch, serum DHT levels remain within the physiologic range during Androderm use.
Another nonscrotal transdermal testosterone delivery system, the Testoderm TTS patch, is available for androgen replacement therapy.13,14 and 15 It is composed of a relatively large (72 cm2) lightly adhesive patch with a reservoir (60 cm2 contact area) containing testosterone in an alcohol-based gel that does not contain permeation enhancers. Nightly application of the Testoderm TTS patch on nonscrotal skin (arms, upper buttocks, or torso) in hypogonadal men results in physiologic serum testosterone and DHT levels with pharmacokinetics similar to that of the Androderm patch. Early clinical experience in hypogonadal men suggests that this patch is associated with less skin irritation but is also less adherent to skin than the permeation-enhanced Androderm patch.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree