ANDROGEN PRODUCTION IN WOMEN
Androgen production in women can be discussed in terms of three separate sources of production: the ovaries and the adrenal glands, which are glandular sources, and the peripheral compartment, which comprises all extrasplanchnic and nonglandular areas of androgen production. The peripheral compartment includes many tissues, the largest of which is the skin. The peripheral compartment modulates androgens produced by the ovaries and the adrenals.
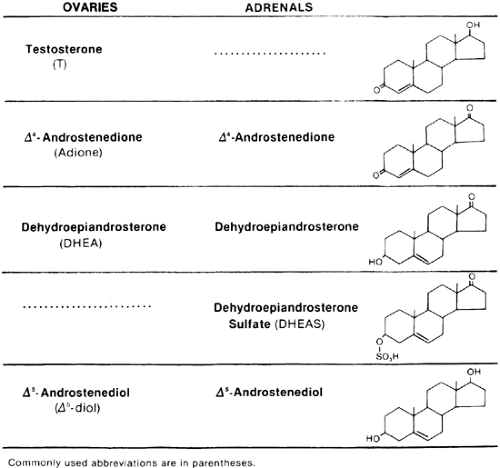
The ovary directly secretes testosterone, Δ4-androstenedione (Adione), and, to a lesser degree, dehydroepiandrosterone (DHEA) (Fig. 101-1). The adrenal gland normally does not secrete testosterone but does secrete Adione in amounts equal to that produced by the ovary in the follicular phase.1 The adrenal gland almost exclusively secretes dehydroepiandrosterone sulfate (DHEAS). DHEAS is secreted systemically in larger quantities than is any other androgen, and its serum concentration correlates significantly with urinary 17-keto-steroid excretion, but it appears to be a more specific marker of adrenal androgen production.2 Another specific marker of adrenal androgen secretion is 11β-hydroxyandrostenedione. Under normal circumstances, the adrenal, but not the ovary, has the ability to 11-hydroxylate. The sensitivity of 11β-hydroxyandrostenedione as an adrenal marker is similar to that of DHEAS, but these two steroids are not correlated and probably reflect different aspects of adrenal androgen production.3 DHEA and Adione are also secreted in greater quantities by the adrenal than by the ovary.4
DIFFERENTIATION OF OVARIAN FROM ADRENAL ANDROGEN SECRETION
Investigators have used various stimulation and suppression protocols to determine the source of androgen production in normal and hyperandrogenic women. Selective catheterization of adrenal and ovarian veins also has been attempted. However, none of these techniques adequately shows the contribution of these glands to the total androgen pool. Selective venous catheterization data have confirmed that almost all exogenously administered agents (e.g., dexamethasone, human chorionic gonadotropin [hCG], adrenocorticotropin [ACTH]) affect the ovary and the adrenal.5 Although these catheterization techniques may be useful in the detection of androgen-producing neoplasms, they have no place in the evaluation of other hyperandrogenic conditions.
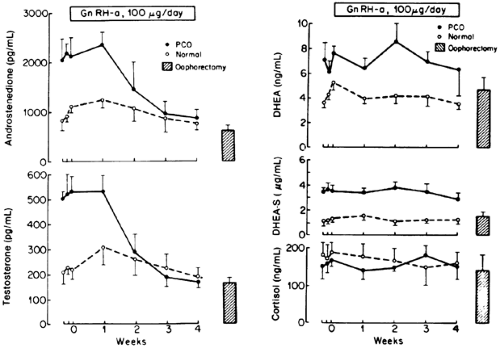
The only probe that has the potential to differentiate ovarian from adrenal androgen secretion is the gonadotropin-releasing hormone (GnRH) agonist (see Chap. 16).6,7 By down-regulating the gonadotrope and the ovary, ovarian androgens (i.e., testosterone and Adione) can be differentiated from adrenal androgens (i.e., DHEA, DHEAS) in normal women and those with polycystic ovary syndrome (PCOS) (Fig. 101-2).Because Adione can be secreted equally by the ovary and adrenal, serum testosterone is the better ovarian marker. Although only one-third of testosterone production (0.25–0.35 mg per day) is secreted directly by the ovary, the remaining two-thirds is derived in near-equal proportions from ovarian and adrenal precursors. Normally, two-thirds of circulating testosterone may originate from the ovary. These concepts, supported by data using a GnRH agonist, allow the conclusion that serum testosterone is the primary marker of ovarian androgen production, and DHEAS and 11β-hydroxyandrostenedione are the best serum markers of adrenal androgen production. However, DHEAS testing is more readily available, and the levels are not subject to diurnal variation.
Unlike in normal ovulatory women, in women with hyperandrogenic disorders such as PCOS, the ovary may contribute to the circulating pool of DHEAS and 11β-hydroxyandrostenedione.8,9 Research using a GnRH agonist has shown that the ovary may be responsible for ˜20% of circulating DHEAS.10 The effort to differentiate ovarian from adrenal androgen secretion is also confounded by the fact that, in most hyperandrogenic conditions, both adrenal and ovarian androgen secretion are increased. In PCOS, 50% to 70% of patients present with adrenal hyperandrogenism,9 whereas in nonclassic 21-hydroxylase deficiency, 50% to 80% present with evidence of ovarian hyperandrogenism.11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree