Fig. 1
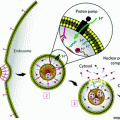
Schematic drawing of radiocatalysis by nanoparticles. Exposure of nanoparticles to ionizing radiation (e.g. X-rays) causes Compton and Photoelectric effects—photoelectrons and Auger electrons (e−) are ejected from the nanoparticles as well as photons of different energies, including secondary fluorescent X-rays. In addition, in semiconductor nanoparticles release of electrons leads to formation of reactive electropositive holes (h+) on nanoparticle surface. Free electrons and electropositive holes on nanoparticle surface can damage cellular components directly or lead to production of reactive oxygen species (superoxide 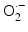 , hydroxyl radical
, hydroxyl radical 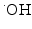 , hydrogen peroxide H2O2) which are also formed by radiolysis of water (adapted from [2, 24, 39])
, hydrogen peroxide H2O2) which are also formed by radiolysis of water (adapted from [2, 24, 39])
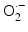 , hydroxyl radical
, hydroxyl radical 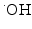 , hydrogen peroxide H2O2) which are also formed by radiolysis of water (adapted from [2, 24, 39])
, hydrogen peroxide H2O2) which are also formed by radiolysis of water (adapted from [2, 24, 39])
Fig. 2
Cellular effects of radiosensitizing nanomaterials. Several different “pathways” to radiosensitization by nanoparticles exist. In the first place, free electrons (released by nanoparticle ionization) are absorbed by surrounding molecules within 15–200 nm from particle surface (e.g. [2, 73]) and ROS (which on average traverse 6 nm distances in cytosol [53]) produced by nanoparticles damage cellular components in addition to incident ionizing radiation and products of water radiolysis. Free electrons and ROS oxidize proteins, peroxidize lipid membranes (cell and organelle membranes both), and produce SSBs and DSBs in DNA [14]. Secondly, nanomaterials may be used to develop hyperthermia under the influence of alternating magnetic field or near infrared light (e.g. [1, 33]). Thirdly, physical interactions between nanomaterials and cellular components, biomolecules, molecular assemblies, and organelles modulate behavior of these cellular elements in different ways leading to such outcomes as perturbations in cell cycle or DNA repair (e.g. [2, 69]). Fourthly, nanomaterials can be made of components (or carry a cargo) specifically designed to make the recipient cells more sensitive to radiation, such as, e.g. delivery of anisense oligonucleotides for DNA repair regulating genes, miRs, or delivery of weak ligands for UPS machinery (e.g. [3, 27, 74])
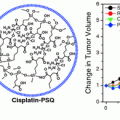
It should also be emphasized that for optimal nanoparticle-mediated radiosensitization, targeting of nanomaterials to specific cell types or subcellular organelles plays a significant role. While selection of best targeting moiety is in and of itself a complicated task, it should be remembered that surface modifications on nanomaterials are not static—nanomaterials accumulate and exchange components of tightly and loosely bound surface protein corona as they progress through cells and their environment (e.g. [40]). According to the “therapeutic ratio” concept, modulating radiation effects on all the cells in the organism equally would provide no advantage over radiation alone if radiation could not be targeted. Therefore, precise in vivo tissue-specific and subcellular delivery of nanomaterials plays a big role in the development of radiosensitizing nanoparticle constructs. However, while (some type) of targeting is absolutely essential for other anticancer nanoparticle formulations (for reviews see e.g. [47]), fortunately that is not so for radiosensitizing nanoconstructs. Ionizing radiation delivery has become so sophisticated that different treatment modalities enable dose delivery closely matching tumor topology. In such a scenario radiosensitizing nanoconstructs would further potentiate such dose differences. In any event, several other chapters of this book discuss nanoparticle targeting more directly, hence this topic will not be specifically addressed here. Rather, this chapter will focus on molecular and biochemical events that occur when nanoparticles are placed in the path of ionizing radiation quality photons. In the interest of brevity, we will not discuss either the basics of radiation physics and radiation biology or the molecular mechanisms relevant for cellular responses to radiation. Much more on these topics can be found in several radiation biology textbooks, e.g. [14, 25]. Finally, the use of nanoparticles for delivery of radionuclides (e.g. [45]) or the delivery of “small molecule” radiosensitizers (e.g. cisplatin [71], paclitaxel [61, 64], docetaxel [63], or curcumin [67]) will also not be covered in this chapter, as this research belongs more appropriately into the topic of “cargo delivery” with nanoparticles, covered conceptually in other chapters of this book. We will touch only very briefly upon nanoconstruct-mediated nucleic acids delivery focusing on those cases where nucleic acids are especially relevant for regulating susceptibility toward ionizing radiation exposure.
Elemental and molecular compositions of nanoparticles discussed in this chapter will include gold, platinum, silver, cerium oxide nanoparticles, gadolinium-doped titanium dioxide and titanium dioxide, iron oxides, copper oxide, gadolinium-carrying nanoparticles, as well as a few polymeric nanoparticles made of elements with small Z values. In response to ionizing radiation elements with higher atomic numbers (greater Z value) produce energetic secondary X-rays, photoelectrons and Auger electrons, while lower Z elements release predominantly Auger electrons; the same is true for nanomaterials made of these materials [2]. Several high Z materials that have been tested as radiosensitizers, e.g. iodine [34] or germanium oxide [29], were found to work as radiosensitizers equally well as single molecules or as nanoparticle formulations.
Finally, we will also speculate about additional, potentially radiosensitizing (but not yet tested with radiation), nanoparticles. One can predict, for example, that nanoparticles that perturb the cell cycle may serve as radiosensitizers. Chemotherapeutic drugs with such properties have been in use for chemoradiotherapy for many years. For example, paclitaxel treatment favors accumulation of cancer cells in G2 and M phases, the most radiation-sensitive phases of the cell cycle, and for that reason it synergistically interacts with ionizing radiation [57]. Incidentally, poly(D,L-lactide-coglycolide)(PLGA) nanoparticles containing taxols are currently being developed as radiosensitizers [18].
1.1 Irradiation in the Presence of Nanomaterials—Effects on Specific (Sub)Cellular Structures and Chemical Milieu
Genomic DNA is the most important target of radiation-induced cellular killing, and therefore we will begin by reviewing work that investigated interactions between DNA and nanoparticles in vitro in the course of exposure to ionizing radiation [73]. The formation of single-strand and double-strand (SSBs and DSBs) DNA breaks in an in vitro setup, using plasmid DNA and gold nanoparticles was used as a proxy for the physicochemical processes governing gold-mediated increase in DNA damage. The basic mechanisms underlying this DNA sensitization were evaluated with electron bombardment with different electron energies [73]. In this study, low electron energies of 1, 10, and 100 eV as well as high energy 60 keV electrons were used. In addition to “standard” DNA lesions such as the SSB and DSB, loss of supercoiled DNA was also used as an index of DNA damage. In this work, considerations for the use of appropriate controls led the authors to compare pure plasmid DNA with “salted DNA” (three Na+ per base) and 1:1 (one particle per plasmid) complexes of DNA with gold nanoparticles (solid gold particles 5 ± 2 nm in size). The yields of all three types of DNA damage (SSB, DSB, and supercoil loss) were enhanced by a factor of 2 or more when plasmid–nanoparticle complexes were compared to the salted DNA sample. This increase was the greatest when 10 eV electrons were used to irradiate DNA–nanoparticle complexes. This finding was explained by the fact that the damage to DNA with 10 eV electrons comes from transient anions forming in the DNA molecule (i.e., the bases, sugar, and phosphate groups) and the gold nanoparticles as well. These anions are resolved either by dissociative electron attachment (DEA), or by deposition of energy into local chemical bonds, resulting in bond breakage. Because DNA damage increased when electrons of 1 and 10 eV are used with nanoparticle–DNA complexes, the authors contended that the gold nanoparticles greatly increase the DEA cross section. Overall, the mechanisms responsible for plasmid DNA damage caused by gold nanoparticles and radiation included: [1] the production of short-range secondary electrons by the gold that has absorbed incoming electrons, with a high probability of interacting with the DNA if the gold particles are in complex with it, and [2] the efficient absorption of low energy electrons by the gold nanoparticles. Moreover, Sanche and collaborators consider these mechanisms critical for gold nanoparticle-dependent DNA damage caused by exposure to X-rays as well, especially for photon energies of 100–110 keV (produced at 100–250 kVp) [73]. It is worth noting, however, that any increase in the distance between the gold nanoparticle surface and plasmid DNA led to a decrease in DNA damage as well. For example, gold nanoparticles coated either with thiolated undecane, dithiolated diethylenetriaminepentaacetic or gadolinium chelating agents reduce (for the same nanoparticle size, particle:plasmid ratio and irradiation conditions) DNA damage enhancement from 2.3 to 1.6 [66].
Using a nucleic acid free system, i.e., gold and silver nanoparticles embedded in an alanine matrix, one recent study used electron spin resonance spectroscopy (ESR) to evaluate dose enhancement in the presence of gold and silver nanoparticles [11]. A conclusion from this work was that, irrespective of X-ray energy, larger and distantly spaced nanoparticles do not cause dose enhancement compared to smaller and closely clustered nanoparticles. Both silver and gold nanoparticles were used in numerous in vitro and in vivo assays (see Sect. 1.2).
In addition to low linear energy transfer (low LET) irradiation (X-rays and gamma rays and free electrons), heavy ion irradiation can be combined with the nanoparticles use as well. Two recent studies by Porcel and others [49, 50] with plasmid DNA and 3 nm platinum nanoparticles coated with polyacrylic acid compared effects of gamma rays with particulate, high linear energy transfer (high LET) irradiation. In this case SSBs and DSBs in plasmid DNA were caused by exposure to gamma rays (with LET = 0.2 keV/μm) and several heavy ions (He2+ LET = 2.3 keV/μm and C6+ LET = 13 keV/μm and LET = 110 keV/μm) alone or in combination with platinum nanoparticles. In all cases nanoparticle addition increased the effects of radiation damage. Use of high Z element (HZE) nanoparticles may be an interesting way to increase the efficiency of high LET radiation treatments.
A comprehensive cell-free study on gold nanoparticles in aqueous solution (without addition of nucleic acids) was also done recently by Misawa and Takahashi [39]. Diagnostic quality X-rays (with a dose rate of 1 Gy/min) in the presence of gold nanoparticles (5–250 nm in diameter) produced increased amounts of reactive oxygen species (ROS), compared to irradiation of aqueous media alone; this increase reached 1.46 for ∙OH and 7.68 for 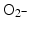 . This finding was explained in part by electron emission of both photo- and Auger electrons and emission of photons of secondary fluorescent X-rays. The authors found that fluorescent X-ray production (which may lead to secondary excitations) and ionization along the X-ray path depended primarily on the volume of the gold particles. More dramatically, however, ROS generation in the presence of gold nanoparticles was inversely proportional to particle diameter indicating that the dominant parameter for ROS production is the surface area of nanoparticles, and not their weight percentage, possibly due to catalytic effects at the particle–water interface. The ROS species evaluated were ∙OH,
. This finding was explained in part by electron emission of both photo- and Auger electrons and emission of photons of secondary fluorescent X-rays. The authors found that fluorescent X-ray production (which may lead to secondary excitations) and ionization along the X-ray path depended primarily on the volume of the gold particles. More dramatically, however, ROS generation in the presence of gold nanoparticles was inversely proportional to particle diameter indicating that the dominant parameter for ROS production is the surface area of nanoparticles, and not their weight percentage, possibly due to catalytic effects at the particle–water interface. The ROS species evaluated were ∙OH,  and 1O2, all of them biologically relevant, with diffusion lengths reaching no more than a few hundreds of nanometers in aqueous media. As mentioned earlier, in a more complex environment of cytosol or nucleosol most ROS progress no more than 6 nm on average [53]. In general, ∙OH species interact with lipids, polysaccharides, proteins, and nucleic acids rapidly and locally.
and 1O2, all of them biologically relevant, with diffusion lengths reaching no more than a few hundreds of nanometers in aqueous media. As mentioned earlier, in a more complex environment of cytosol or nucleosol most ROS progress no more than 6 nm on average [53]. In general, ∙OH species interact with lipids, polysaccharides, proteins, and nucleic acids rapidly and locally. 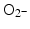 radicals react more slowly with lipids, however, in the presence of iron and hydrogen peroxide these radicals can lead to production of ∙OH radicals (these chemical processes are described by Haber–Weiss and Fenton reactions). Finally, 1O2 radicals react with unsaturated fatty acids, beta-carotene, amino acids, and methionine. Each one of these interactions damages biomolecules and causes stress than can lead to cell death or genomic instability.
radicals react more slowly with lipids, however, in the presence of iron and hydrogen peroxide these radicals can lead to production of ∙OH radicals (these chemical processes are described by Haber–Weiss and Fenton reactions). Finally, 1O2 radicals react with unsaturated fatty acids, beta-carotene, amino acids, and methionine. Each one of these interactions damages biomolecules and causes stress than can lead to cell death or genomic instability.
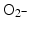 . This finding was explained in part by electron emission of both photo- and Auger electrons and emission of photons of secondary fluorescent X-rays. The authors found that fluorescent X-ray production (which may lead to secondary excitations) and ionization along the X-ray path depended primarily on the volume of the gold particles. More dramatically, however, ROS generation in the presence of gold nanoparticles was inversely proportional to particle diameter indicating that the dominant parameter for ROS production is the surface area of nanoparticles, and not their weight percentage, possibly due to catalytic effects at the particle–water interface. The ROS species evaluated were ∙OH,
. This finding was explained in part by electron emission of both photo- and Auger electrons and emission of photons of secondary fluorescent X-rays. The authors found that fluorescent X-ray production (which may lead to secondary excitations) and ionization along the X-ray path depended primarily on the volume of the gold particles. More dramatically, however, ROS generation in the presence of gold nanoparticles was inversely proportional to particle diameter indicating that the dominant parameter for ROS production is the surface area of nanoparticles, and not their weight percentage, possibly due to catalytic effects at the particle–water interface. The ROS species evaluated were ∙OH, 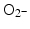 and 1O2, all of them biologically relevant, with diffusion lengths reaching no more than a few hundreds of nanometers in aqueous media. As mentioned earlier, in a more complex environment of cytosol or nucleosol most ROS progress no more than 6 nm on average [53]. In general, ∙OH species interact with lipids, polysaccharides, proteins, and nucleic acids rapidly and locally.
and 1O2, all of them biologically relevant, with diffusion lengths reaching no more than a few hundreds of nanometers in aqueous media. As mentioned earlier, in a more complex environment of cytosol or nucleosol most ROS progress no more than 6 nm on average [53]. In general, ∙OH species interact with lipids, polysaccharides, proteins, and nucleic acids rapidly and locally. 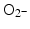 radicals react more slowly with lipids, however, in the presence of iron and hydrogen peroxide these radicals can lead to production of ∙OH radicals (these chemical processes are described by Haber–Weiss and Fenton reactions). Finally, 1O2 radicals react with unsaturated fatty acids, beta-carotene, amino acids, and methionine. Each one of these interactions damages biomolecules and causes stress than can lead to cell death or genomic instability.
radicals react more slowly with lipids, however, in the presence of iron and hydrogen peroxide these radicals can lead to production of ∙OH radicals (these chemical processes are described by Haber–Weiss and Fenton reactions). Finally, 1O2 radicals react with unsaturated fatty acids, beta-carotene, amino acids, and methionine. Each one of these interactions damages biomolecules and causes stress than can lead to cell death or genomic instability.1.2 Irradiation in the Presence of Nanomaterials—Effects on Cells in Vitro and/or Tumors in Vivo
While it is known that irradiation of gold nanoparticles in complexes with DNA leads to DNA damage, the effects of gold nanoparticles on whole cells are far more complex and have rarely been fully investigated in a single research paper. Most frequently, in cellular in vitro studies changes in cell viability are the primary endpoint of investigation, and the exact mode of action of nanoparticles has received relatively little attention. A few examples of such work (see Sect. 2) suggest that biochemical/biological effects of gold nanoparticles play perhaps an even greater role in radiosensitization than the production of secondary electrons.
In an effort to investigate the exact radiation parameters for increased radiation-dependent cell killing in vitro in the presence of gold nanoparticles, Rahman and others [51] used 1.9 nm gold nanoparticles (mixed at 0.197 mg/ml) with bovine aortic cells and exposed such cells to monochromatic, synchrotron-generated X-rays of 30, 40, 50, 60, 70, 81, and 100 keV. The greatest dose enhancement in cell killing was found for the combination of gold nanoparticles with 40 keV X-rays, followed by 50 and 60 keV dose enhancement values. Gold nanoparticles were also tested in combination with proton irradiation [48], and increased cell killing was found in samples of cells that had internalized the nanoparticles. (It should be noted that prolific ROS production outside of cells but close to cellular membrane can lead to cell death as well.)
Increased efficiency of radiation was also noted when radioactive seed therapy (I-125 brachytherapy seeds) was combined with gold nanoparticles [43]. Dose ranges varied between 2.1 and 4.5 cGy/h with an even dose distribution in adherent layers of HeLa cells. These cells were treated with 50 nm gold nanoparticles, and the number of gamma H2AX foci (signifying DNA DSBs) following irradiation in nanoparticle treated cells was 1.7–2.3 fold greater compared to controls.
Nanoconstructs prepared by simple one-pot synthesis as silver nanoparticles coated with egg white proteins were used to radiosenistize breast adenocarcinoma cells [30]; increasing concentration of nanoparticles with a fixed (4 Gy) irradiation led to increased cell killing even though no cytotoxicity was recorded in the absence of radiation, even at the maximal concentration of conjugates (12 mg/L).
Silver was also used to make the shell on the surface of carbon nanodots [24]. These nanoconstructs (5–100 nm in size including polyethylene glycol (PEG) coating) were tested in vitro both for photodynamic therapy and as radiosensitizers. Reduction in cell survival of Du145 prostate cancer cells with combination treatment, compared to radiation alone, was twofold with 15 Gy.
A growing body of literature shows promising data on nanoparticle-mediated radiosensitization in vivo. For example, syngeneic EMT-6 mammary tumors implanted subcutaneously into the hind limb of Balb/c mice and injected intravenously with 1.9 nm gold nanoparticles were irradiated 2 min later with 110 keV X-rays (produced at 250 KVp) by Hainfeld and others [12]. At gold concentration of 2.7 g/kg and 26 Gy dose complete tumor regression for 1 year was obtained for 86 % of mice compared to 20 % of mice given irradiation alone. In their more recent work, the same group of investigators used 11 nm gold nanoparticles administered intravenously at 4 g/kg to B6C3F1 mice bearing syngeneic orthotopic Tu-2449 brain tumors. Fifteen hours after nanoparticle administration, these mice were exposed to local 30 Gy (at 100 KVp) irradiation. A year after treatment 50 % of mice exposed to radiation in the presence of nanoparticles were still alive [13].
Among the nanomaterials that radiosensitize cells by potentiating the physicochemical effects of radiation is cerium oxide. Cerium oxide particles (5–8 nm) engage in different chemical reactions with different reactive oxygen species in a pH-dependent manner. At acidic pH, cerium oxide nanoparticles scavenge superoxide radical and produce hydrogen peroxide. At neutral pH values, cerium oxide nanoparticles scavenge H2O2 in a chemical reaction resembling the decomposition of hydrogen peroxide to water and oxygen mediated by catalase [62]. This pH-dependent behavior of cerium oxide leads to radioprotection of healthy tissues and cells living under “neutral pH” conditions, and confers radiosensitization of cancer cells that are characterized by an acidic intracellular milieu. This was shown not only with clonogenic assays in vitro, but also in vivo, using mice carrying orthotopic pancreatic tumor (human cell line L2.6pl) xenografts. While nanoparticles alone delivered intraperitonealy (IP) caused tumor volume reduction and apoptotic cell death as determined by TUNEL staining, administration of cerium oxide nanoparticles prior to ionizing radiation lead to increased tumor regression and enhanced apoptotic cell death [62]. As the production of ROS in acidic subcellular environment could lead to increased toxicity, one of the potentially interesting approaches to increase the usability of cerium oxide nanoparticles is to target them to acidic lysosomes by covering them with a negative surface charge [8].
Increased production of ROS and the subsequent increase of cell death monitored by in vitro assays were found both with gold nanoparticles and nanoparticles made of other materials. For example, lanthanide-doped titanium oxide nanoparticles increased radiation-induced cell death of several human cell lines [59]. Levels of radiosensitization differed from cell type to cell type (embryonal rhabdomyosarcoma cell lines RD, alveolar rhabdomyosarcoma RH30 and breast cancer cell line MCF7), suggesting that the cellular capacity for nanoparticle uptake as well the ability to cope with ROS burdens play very significant roles in any radiosensitization by means of nanomaterials. The same nanoparticle type (silica coated and rare earth doped TiO2 crystals 3–10 nm in size) was injected into tumors in vivo. Tumor-bearing mice were irradiated with 10 fractions of 2.5 Gy followed by 3 fractions of 2 Gy over the same period [60]. Hind limb xenograft tumors grown in SCID mice were human lung adenocarcinoma cell line A459; in the presence of nanoparticles (three injections of 50 ml of 1 mg/ml nanoparticles each) an additional twofold reduction of tumor volume upon radiation was found.
An interesting research direction with regard to rare earth elements and nanoparticle-based radiosensitization is the use of gadolinium-based nanoparticles [5, 36, 37]. These 2.9 nm particles consist of a polysiloxane core and gadolinium–DTPA chelates (with cyanine 5.5 added to allow optical imaging) and have been tested first in orthotopically implanted 9L gliosarcoma (9LGS) brain tumors in Fisher 344 rats. Following intravenous delivery these nanoconstructs had good renal excretion and could be used both for magnetic resonance imaging and radiosensitization [5]. The importance of timing between nanoparticle delivery and irradiation (delivered with a microbeam) was very noticeable in this study; a 5 min delay increased radiation damage to normal tissue and decreased overall survival of rats compared to radiation alone, while a 20 min delay lead to improved survival of animals compared to radiation alone. A recent study using the same type of nanoparticles in xenograft head and neck cancer models (subcutaneous implantation of human head and neck cancer cell line SQ20B into flank of nude mice) showed that intratumor injection of gadolinium nanoparticles followed immediately with 10 Gy dose of ionizing radiation had a synergistic effect on tumor size reduction [37]. In vitro experiments with the same cell line as well as FaDu and Cal33 head and neck carcinoma cell lines, demonstrated that the use of gadolinium nanoparticles was associated with an increase in nonreparable DNA DSBs and the shortening of G2/M arrest, leading to increased genomic instability, appearance of polyploid and then hypoploid cell populations and increased apoptosis.
It should also be noted that radiation itself can alter delivery of nanomaterials. For example, Joh et al. [19] found that exposure to ionizing radiation increases the permeability of the neovasculature for nanoparticles. When nude mice orthotopically inocculated with the human glioma cell line U251 received 20 Gy (focused through a 1.5 cm collimator of the small animal irradiator) prior to injection of 22 nm PEG-coated gold nanoparticles, increased extravasation and in-tumor deposition of nanoparticles occurred, suggesting that radiation can improve the passive tumor tissue targeting of nanoparticles [19]. Radiation induced a three-fold increase of enhanced permeability retention in irradiated versus nonirradiated tumors.
2 Modulation of Radiosensitivity by Hyperthermia
Modulation of temperature conditions in the body (especially increased tumor temperature) at the time of radiation treatment has been noted to be a potential radiosensitizer, as reviewed by Wust and others [65]. Though hyperthermia was used in the treatment of various diseases including cancer since ancient times, one of the first carefully recorded local applications of hyperthermia alone for cancer treatment was performed in 1898 by Swedish gynecologist Westermark, who treated cervical cancer by running hot water through an intracavitary spiral tube and noted an excellent clinical response in seven patients [41].
On a molecular and cellular level, temperatures of 40–45 °C trigger various cellular responses, including protein denaturation [22], alterations in the cytoskeleton and membrane [15] and cell death and permanent arrest [22]. Temperatures equal to or higher than the transition temperature of 42.5 °C are considered to be optimal for generating protein damage to cells, but these temperatures are difficult to achieve in vivo [14]. In combination with ionizing radiation, hyperthermia is particularly potent predominantly because the two have different targets, with radiation damaging DNA and hyperthermia damaging proteins. For example, temperatures near the transition temperature (42 °C) increase tumor vascular perfusion [15] leading to an increase in tumor oxygenation, which increases the efficiency of radiation as formulated by oxygen enhancement ratio (OER). Protein denaturation and aggregation in the nucleus caused by hyperthermia perturb DNA synthesis and repair [23]. Regardless of the precise molecular mechanisms, chemo- and radiosensitizing effects of hyperthermia are significant, and hyperthermia treatment is considered to be among the most potent radiosensitizers discovered to date. Furthermore, thermal radiosensitization appears to be most pronounced in cells that are hypoxic and those that are in S phase, i.e., in those cells that tend to be the most resistant to radiotherapy alone [15].
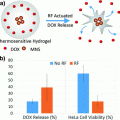
Magnetic nanoparticles in combination with magnetic field exposure can be used to induce a site-specific hyperthermia [70]. After localizing in a tumor, magnetic nanoparticles can be activated by an alternating magnetic field (AMF), causing a local increase in temperature. Such nanoconstructs can heat their immediate submicron environment through eddy currents (circular electric currents induced within conductors by a changing magnetic field), magnetic hysteresis, and Neel or Brownian relaxation (complex physical phenomena that depend on the size of the particles). There is also evidence of additive magnetic effects via electromagnetic coupling through nanoconstruct aggregation either extracellularly or in endosomes [21, 56, 70]. This is most likely caused by the increase in “aggregated size” of magnetic material. Deliberately designed magnetic nanoparticle assemblies, such as, e.g. magnetic nanoparticle–adenovirus assemblies, have an increased capacity for thermal ablation [70].
Three recent studies illustrate the promise for magnetic nanoparticles-dependent hyperthermia-induced radiosensitization. The first study involved nanoparticles with a hematite core and hydrodynamic diameter of 117 nm injected into syngeneic mouse breast cancer tumors implanted into hind limb of C3H/He mice. Tumors were first subjected to AMF at 169 kHz or microwave exposure and then to irradiation—15 Gy of 6 MeV electrons within 30 min of hyperthermia. While tumor volume tripling time after the best single treatment was 18.7–25 days, combination therapy showed a tumor tripling time of 42.6 days [9]. A more recent study by Lin and others used Mn0.5Zn0.5Fe2O4 nanoparticles coated with poly(ethyleneimine) to deliver gene therapy [28]. The DNA construct was a combination of a promoter region of radiation responsive early growth response protein 1 (Egr-1) and the “suicide gene complex” HSV-TK/GCV. Expression of herpes simplex virus thymidine kinase (HSV-TK) converts the prodrug ganciclovir (GCV) into GCV triphosphate, a DNA synthesis and cell cycle inhibitor. In a hepatoma xenograft model (HepG2 tumors in BALB/c nude mice) use of these nanoconstructs with induction of hyperthermia and combined with irradiation lead to tumor volume decrease greater than 90 %, two times better than radiotherapy alone [28].
The final study to be mentioned here is a phase II clinical trial using magnetic nanoparticles in 66 human patients, 59 with recurrent glioblastoma multiforme. Nanoparticles consisted of 12 nm magnetite with aminosilane coating, at iron concentration of 112 mg/ml, injected directly into tumors. Six one-hour long hyperthermia sessions (induced by AMF) were done semiweekly, with median tumor temperature of 52 °C. These sessions were immediately preceded or followed with stereotactic beam radiotherapy (SBRT) with a median dose 30 Gy, delivered as five 2 Gy fractions each week. The primary study endpoint was overall survival (OS) after recurrence—it reached 13.4 months, which is a significant improvement compared to, e.g. 6.2 months recoded in a metastudy on temozolomide as a salvage treatment [33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree