Fig. 1
Overlap between Cushing’s syndrome and metabolic syndrome
Although the example of Cushing’s syndrome makes a link between cortisol and obesity in the general population theoretically plausible, it represents an extreme example of chronically high cortisol exposure which does not occur in normal physiology. Further evidence that cortisol may have an adverse effect on the cardiometabolic phenotype and adiposity stems from studies investigating sensitivity to cortisol. Cortisol exerts its effects by binding to the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR). Most of the metabolic effects of cortisol, including the effects on body composition leading to truncal obesity, are thought to arise from gene transactivation by the GR after ligand binding [19]. Over the past two decades, several polymorphisms in the GR have been described that influence the sensitivity to glucocorticoid s . Approximately half of the general population carries either the N363S or BcII polymorphism, both of which have been associated with an increased sensitivity to glucocorticoids [20, 21]. Carriage of either of these variants has been associated with adiposity, supporting the concept that an increased activity of cortisol at the tissue levels promotes obesity [22–24]. In contrast, the ER22/23EK polymorphism , which is carried by about 8–9 % of the population, is associated with a relative resistance to glucocorticoids [25–27]. ER22/23EK carriers appear to be relatively protected against the deleterious cardiometabolic effects of cortisol, exemplified by increased lean body mass and insulin sensitivity, and lower cholesterol levels [26, 27].
There have been numerous attempts to unravel the association between obesity and exposure to systemic cortisol levels, using measurements in urine, saliva and blood. To interpret the results of these studies, it is important to take note of several situational and physiological factors that influence cortisol measurements. Cortisol follows a diurnal rhythm, characterized by a peak in the early morning (the cortisol awakening response, CAR) and generally declining levels during the day. Cortisol rises in response to physical or psychological factors, which causes cortisol levels to be variable within and across days [28]. Saliva and blood measurements can be used to obtain information about time-point cortisol levels, while urinary free cortisol (UFC) is used to estimate the total cortisol output over a 24-h period [18, 28].
A recent systemic review highlighted that studies investigating the associations between obesity and cortisol in body fluids provide inconsistent results [29]. Most published studies indicate that obesity is characterized by a diurnal rhythm with a blunted cortisol awakening response and a less sharp decline in cortisol levels over the course of the day. 24-h UFC tends to be higher in obese individuals, and the cortisol reactivity to acute stressors appears to be exaggerated. In most cases, negative studies or even opposing results have been reported as well [29]. These apparently inconsistent results may not be surprising, when we take into account the high variability of cortisol levels (Fig. 2) .
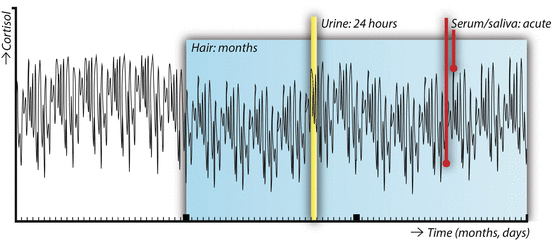

Fig. 2
Conceptual overview of the different matrices in which cortisol can be assessed: serum and saliva (time-point), urine (intermediate term output), and scalp hair (long-term cumulative levels). The line depicting circulating cortisol levels over a period of 3 months is fictional
Measuring Long-Term Exposure to Cortisol : Hair Analysis
A relatively novel way to account for the high variability in cortisol levels is scalp hair analysis . Scalp hair grows at a relatively stable rate of about 1 cm per month. During hair growth, substances are incorporated into the hair. This makes hair a suitable matrix to retrospectively assess long-term exposure to substances, depending on the length of the hair, up to several months back in time. Over the past decades, hair analysis has become an established method to retrospectively examine exposure to drugs of abuse and environmental toxins [30].
The first published report of endogenous glucocorticoids measured in human hair dates back to 2004 [31]. Scalp hair steroid analysis has since been performed in a number of labs and has greatly expanded the time frame of cortisol exposure that can be examined in a single measurement, as shown in Fig. 2. It is assumed that circulating free steroid hormones diffuse from the bloodstream into the hair shaft, although there may be minor contributions from sebum and sweat as well. Although, at first, in most studies immunoassays were used to measure cortisol, more recent studies report both hair cortisol (F) and cortisone (E) analyzed using liquid chromatography–tandem mass spectrometry (LC-MS/MS) . Besides offering information about multiple simultaneously measured steroids, LC-MS/MS has higher sensitivity and is not hindered by antibody cross reactivity [32].
In the past decade, hair analysis has been used to measure long-term cortisol (and sometimes cortisone), most often in hair segments of 3 cm length, corresponding to cumulative levels over a period of 3 months.
In both obese adults and children, we found that hair cortisol concentrations (HCC) were increased compared to nonobese controls [33, 34]. Furthermore, in the largest population-based studies, HCC were positively associated with BMI and waist circumference, indicating that long-term cortisol exposure is on average increased in adiposity [35, 36]. Furthermore, increased HCC have been associated with presence of MetS and its separate components in a middle-aged population, as well as diabetes mellitus and cardiovascular disease presence in elderly populations (Table 1) [36–38].
Table 1
Published associations between health and situational factor s and hair cortisol levels (adapted with permission from: Wester and van Rossum, Eur J Endocrinol 2015 [32])
Increased hair cortisol | Decreased hair cortisol | |
|---|---|---|
Somatic health factors | Cushing’s syndrome | Childhood asthma with inhalation glucocorticoids |
Hydrocortisone use | ||
Obesity | ||
Metabolic syndrome | ||
Diabetes mellitus | ||
Cardiovascular disease | ||
Heart failure severity | ||
Recent myocardial infarction | ||
Chronic and acute stressors | Intensive aerobic exercise | Traumatic experience |
Trauma | ||
Life events | ||
Unemployment | ||
Shift work | ||
Severe chronic pain | ||
Psychopathology | Posttraumatic stress disordera | Posttraumatic stress disordera |
Major depressive disorder | Generalized anxiety disorder | |
Bipolar disorder, late onset | Panic disorder |
Besides cardiometabolic parameters, a range of other clinical and situational factors have been investigated in relation to long-term cortisol levels. In larger studies, hair cortisol levels are higher in men and increase with age. Various hair-related parameters are also associated with hair cortisol and should be considered in clinical studies as potential confounders (Table 2) [32, 35].
Table 2
Overview of demographic and confounding factors that (potentially) affect hair cortisol concentrations
Factor | Significance for hair cortisol levels |
|---|---|
Age | Increase with age |
Sex | Higher in males |
Season | Spring and summer may increase levels |
Hair treatment | Inconsistent results |
Hair washing frequency | Slightly lower with higher hair washing frequency |
Sweating on the scalp | Experimental evidence is mixed |
Use of corticosteroids | Both lower and higher hair cortisol levels have been reported; dependent on the corticosteroid and used method |
Mood and anxiety disorders have been associated with alterations in hair cortisol levels (Table 1) [39]. Psychosocial stress, measured using standardized questionnaires such as the Perceived Stress Scale, has to date not been consistently associated with hair cortisol concentrations [32]. However, exposure to several physical and mental stressors has been associated with increases, including chronic pain, intensive aerobic exercise, and major life events (Table 1) [40–43]. This suggests that it may be the stressor itself, more than the subjectively experienced stress level that is associated with an increase in HPA axis activity .
Future Directions and Unresolved Issues
The studies involving scalp hair cortisol support the concept that obesity and its adverse cardiometabolic risk profile are associated with an increase in long-term systemic cortisol exposure. Whether this subtle hypercortisolism contributes to the development of obesity, insulin resistance, dyslipidemia, and cardiovascular disease is unknown, but it is likely from a pathophysiological perspective. Longitudinal studies may shed further light on this issue and determine whether hair glucocorticoid measurements deserve a place in cardiovascular risk stratification.
Obesity is known to be associated with increased psychological distress, social stigma, and psychopathology [44, 45]. This may explain part of the relationship between long-term cortisol and obesity. However, the evidence to date indicates that the subjective perception of stress has little impact on long-term cortisol levels [32]. Perhaps this association is modulated by individual factors, and only prone individuals suffer from increased long-term cortisol exposure. Furthermore, cortisol metabolism may be altered in obese individuals. Cortisol is primarily metabolized in the liver, and nonalcoholic fatty liver disease associated with obesity may influence cortisol metabolism [46]. Additionally, obesity is associated with low-grade inflammation, which may increase cortisol levels [47]. However, even if increased cortisol follows, rather than precedes cardiometabolic derangements, it is likely to at least contribute to the maintenance of an unfavorable risk profile.
The fact that hair collection is easily applicable in a clinical practice or research setting, with minimal burden to the participant, makes this method ideal to study the effects of behavioral, medical, or surgical interventions on long-term glucocorticoid exposure. Well-designed intervention studies involving hair analysis may greatly improve our understanding of the role of subtle variations in chronic HPA axis activity in health and disease, possibly paving the way to a more tailored treatment of obesity and cardiometabolic risk.
Several mechanistic questions regarding hair cortisol remain unresolved. It is assumed that steroids can incorporate into the hair through passive diffusion from the circulation, but there may also be contributions from sweating and sebum. The relative contribution of these three mechanisms is currently not known. Although sweating challenges do not seem to acutely influence hair cortisol, it is conceivable that repeated sweating over prolonged periods of time may affect hair levels measured [48]. Furthermore, the influence of conversion from cortisol to cortisone and vice versa by 11 beta hydroxysteroid dehydrogenases (11β-HSD) on hair cortisol and cortisone is not fully understood. Both local (e.g., skin or hair follicle) and overall systemic 11β-HSD could theoretically impact the ratio between cortisol and cortisone in hair [49]. At present, methods are available that measure both hair cortisol and cortisone using LC-MS/MS, yielding the potential to explore the ratio between these two as a marker for systemic 11β-HSD activity [50, 51]. We expect that both experimental and epidemiological studies may help understand how glucocorticoids are incorporated into the hair, as well as unravel the contribution of peaks in circulating hormone levels and local regulation to hair glucocorticoids .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree