1.2 Genotypes
To date, more than 150 human papillomavirus (HPV) types have been completely sequenced. The HPV types linked to anogenital and oropharyngeal cancers are generally categorized as high-risk and low-risk types based on their association with human cancer on epidemiological studies or on their evolutionary profile [3, 4]. Among all potential high-risk types, 12 HPVs (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) are classified by the International Agency for Research on Cancer (IARC) as carcinogenic to humans (category 1); HPV 68 is categorized as probably carcinogenic (Group 2A) and HPV types 26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85, and 97 as possibly carcinogenic (Group 2B) [5]. Among cervical cancer cases, 96.9 % will harbor types belonging to the IARC Group 1, and over 70 % of cervical cancers will be attributable to HPV 16 and/or 18 [6, 7]. In sites other than cervix, HPV 16 tends to play even a higher contribution (Table 1).
Table 16.1
HPV types according to the level of evidence of carcinogenicity for human cancer
HPV broad risk categories | International Agency for Research on Cancer (Vol 100B) | HPV type | Relative contribution in cervical cancer (%)d |
|---|---|---|---|
High risk | Group I Carcinogenic to humans | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 | 96.9 |
Group 2A Probably carcinogenic to humans | 68 | 0.7 | |
Group 2B Possibly carcinogenic to humans | 26, 53, 66, 73, 82 | 1.3 | |
30, 34, 69, 85, 97a | 0.5 | ||
5, 8b | 0 | ||
Low risk | Group 3 | 6, 11c | 0.1 |
2 HPV Acquisition and Persistence in the Cervical Mucus
Mucosal HPV infection in the cervical mucus is very common and largely acquired through sexual activity. As such, HPV is highly prevalent in the years around sexual initiation decreasing thereafter as a consequence of acquired immune response and a decrease of sexual exposure of new partners with age [9, 10]. When data on women of all ages and with no disease are combined, the estimated point prevalence of HPV infections worldwide is around 11–12 % (Fig. 3). The estimates are higher in sub-Saharan Africa (24 %), Eastern Europe (21 %), and Latin America (16 %). Lower estimates are seen in Northern Africa (9 %) and Western Asia (2 %). These geographical differences can largely be explained by variations of age at sexual initiation and the average number of sexual partners in the population. The study of HPV prevalence by age group is an excellent surrogate of the sexual behavior in the community which in turn can be useful when planning preventive strategies such as best age at vaccination or screening strategies based on HPV DNA detection. As an example, one could compare the HPV DNA detection in vaginal samples in young girls in two contrasting countries in terms of cervical cancer incidence. In Tanzania, a country with very high incidence rate of cervical cancer, 36 % of girls aged ≤16 years were already HPV positive; the prevalence increased to 86 % in 19–20-year-olds and then declined to 64 % in those aged >23. In a similar period in Spain, a country with low incidence of cervical cancer, HPV picked at 29.9 % at age 19 [11–13]. After 12 months of follow-up of the young girls in Tanzania, only 27.2 % of those originally detected to harbor HPV infection remained positive, in agreement with other prospective studies [14].
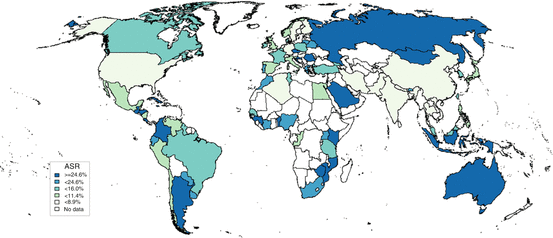

Fig. 16.3
Prevalence of HPV among women with normal cytology in the World Data updated at 15 Dec 2014 (data as of 31 Oct 2014). The samples for HPV testing come from cervical specimens (fresh/fixed biopsies or exfoliated cells. (Ref: http://www.hpvcentre.net)
Contrary, infections seen in women in the middle ages are more likely to be prevalent as new infection acquisition is less prominent at these ages [15]. The fraction of persistent carriers of HPV in this age group is estimated in a range of 4–10 %, and these women are the true high-risk group for cervical cancer.
Interestingly, HPV in postmenopausal women shows important variations in different geographies. While worldwide HPV reaches its lowest estimates after age 50, it is common to observe in some studies a second rebound of the prevalence. It is unclear what drives this second peak, but the lack of a universal pattern seems to indicate that it must relate to either behavioral aspects (i.e., acquisition of new partners or use of local estrogens) or to a cohort effect where the overall pattern reflects the sum of several cohorts with different background HPV prevalence.
In all these age-related scenarios of HPV infection, the time lag between the peak of HPV infection and the peak of cancer incidence is around two to four decades, making the initiating infections and precursor lesions of cervical cancer an appropriate target for screening and early detection.
Unfortunately, the mechanisms related to the process to clearance or persistent infection are still unknown. Host, virological, and behavioral factors may all play a role. Probably, the best recognized factor is the higher persistence associated to HPV 16 infection compared to other HPV types [14]. Some screening strategies now recommend to have a distinctive follow-up of women that screen positive for HPV 16/18 as compared to other types [16].
2.1 Non-viral
Several cofactors have long been proposed, such as smoking and parity that determine whether a woman develops CIN3 and eventual cervical cancer. Diet and coinfection with other STI (i.e., HIV, Chlamydia trachomatis) are also likely to play a role. Host immunity is an obviously important but difficult-to-study etiologic factor. Condom use is partially protective from initial infection; however, its role in clearance of persistent infections is less clear [14].
HIV infection is probably one of the most relevant cofactors for cervical carcinogenesis [17, 18]. Women infected with HIV had higher prevalence of HPV infection, persistent infection with HPV, infection with multiple types of HPV, and higher prevalence of cervical cancer precursors. In a study with South African women, HIV seroconversion almost doubled the HPV prevalence after seroconversion and showed increased risk of low-grade cytological abnormalities compared with HIV-negative women [19]. However, data on 241 HIV-positive women with ICC, mainly from sub-Saharan Africa, suggest that the combined prevalence of HPV16 and/or 18 is 68 %, i.e., not different from the prevalence found in HIV-negative women with the same disease in the same region.
3 HPV and Cervical Cancer
Cancer of the cervix uteri is the fourth most common cancer among women worldwide, with an estimated 527,624 new cases and 265,653 deaths in 2012. This represents 4.1 % of the total cancers diagnosed in the world every year and 75 % of all HPV-related cancer sites. Worldwide, mortality rates of cervical cancer are substantially lower than incidence with a ratio of mortality to incidence to 50.3 % [20]. The majority of cases are squamous cell carcinoma followed by adenocarcinomas [21, 22].
The first signs of an HPV infection are morphologically recognized as cervical intraepithelial lesions grade 1 or low-grade lesions (CIN1/LSIL). These have a high potential to regress, and no treatment is recommended. If the infection persists, the cellular changes become more generalized and pronounced leading to CIN 2/3 or high-grade lesions (HSIL). These lesions, generally asymptomatic, are detectable through screening and require treatment as their regression potential is very low [23]. In a very small proportion of women, the HPV infection can evolve ultimately inducing invasive cervical cancer. In this case, women show up with clinical symptoms such as abnormal bleeding, abdominal pain, or coital pain. The time from infection to invasive cervical cancer can be that of decades and is extremely rare to observe cancer cases before the age of 30. In screened populations, it is however common to detect CIN2–3 lesions in women younger than 30 years old which may favor overtreatment of regressing lesions.
Case-control studies exploring the risk to develop invasive cervical cancer associated to individual HPV types showed that the magnitude of the risk can be over 200 times higher for some high-risk types like HPV 16 and HPV 18 compared to uninfected women of same age [4]. As it can be seen in Fig. 4, only HPV 6 and HPV 11 showed a non-statistically significant increased risk. This is in agreement with the rare observation of these types as cause of human cancer.
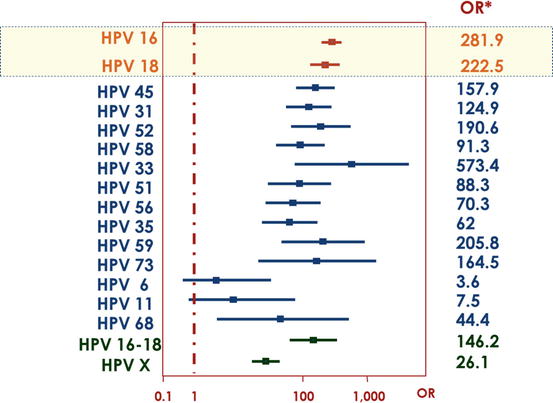

Fig. 16.4
Odds ratios and 95 % confidence intervals of invasive cervical cancer by individual genotypes [4]
HPV is recognized to be the necessary cause for cervical cancer development of epithelial origin, well accommodating the established causality rules [24, 25]. Over 90 % of the cervical cancer cases not only harbor viral HPV DNA but also show detection of transcripts encoding the viral E6 and E7 oncoproteins supporting the cell transformation step necessary for carcinogenesis [26, 27]. The HPV-type distribution in cervical cancer specimens has been extensively studied [4, 6, 28]. Worldwide, HPV 16 and 18 contribute over 70 % of ICC cases, and HPV 16, 18, and 45 can explain over 90 % of cervical adenocarcinomas. Globally, HPV 16, 18, or 45 ICC cases are reported to be diagnosed at younger ages than other HPV types [6, 29].
Few exceptions are documented in which cervical cancer tissue of epithelial origin is HPV negative after applying different HPV assays. In particular, HPV-negative cases are identified in some subtypes of the adenocarcinoma category [30, 31]. There are no large series of adenocarcinoma samples using fresh tissue where DNA is easily detected. However, a series of 682 paraffin-embedded tissue samples of adenocarcinomas has been recently evaluated in HPV detection after careful pathology review aiming to exclude misdiagnosis of endometrial tumors. In the series, cases classified as classic cervical adenocarcinoma type contributing 83 % of the total showed high HPV positivity (71.8 %), while the other less common subtypes had significantly lower HPV prevalence (endometrioid 27.3 %, serous 25 %, clear cell 20 %, not otherwise specified 13.9 %, and minimal deviation 8.3 %). Although a different etiological pathway cannot be dismissed for rare adenocarcinoma subtypes, negativity was related to region, advanced patient’s age, and longer sample storage time. These cases can be considered anecdotal and do not suggest any major change of our understanding of cervical carcinogenesis.
3.1 Screening
Cervical cancer persistently shows a social inequality pattern by which the poorer have higher rates of disease [32]. This social gap between and within countries is largely attributable to poor screening uptake. Cervical cancer takes decades to develop, and early detection of preneoplastic lesions is a major asset for cancer prevention. Regular exam of cervical exfoliates through cytology or HPV detection has been shown to decrease mortality of cervical cancer [33–35]. Unfortunately, few women in the world have a regular access to screening [36]. While in the Western Europe over 70 % are regular users of screening, in sub-Saharan Africa less than 5 % of the women have ever been screened. In developed countries, invasive cervical cancer mainly arises in non-screened women pointing to the relevance of reaching high population coverage to impact incidence and mortality of disease.
4 HPV and Vulvar, Vaginal, Anal, and Penile Cancers
Cancer incidence rates at anogenital anatomical sites other than cervical cancer are much lower than that observed for cervical cancer. Vulvar cancer is a rare entity with age-adjusted incidence rates ranging from 0 to 4.6 per 100,000 women-year, representing about 4 % of all gynecological malignancies. It is estimated that each year, about 27,000 new cases are being diagnosed worldwide [7, 21]. Lower rates are observed in Asia and Africa than in other parts of the world. Over the past few decades, the incidence rates of invasive vulvar cancer (IVC) and vulvar intraepithelial neoplasia (VIN) have both been reported to increase, particularly among younger women. However, in the United States, the increase is limited to preneoplastic lesions, and in the United Kingdom, trends are stable [37].
Squamous cell carcinoma (SCC) accounts for more than 90 % of the malignant tumors of the vulva. The basaloid and warty histological variants, representing about 1/3 of cases, are common in younger women and are often associated with HPV DNA detection. These tumors share many risk factors with cervical cancer. By contrast, keratinizing variants arise from chronic vulvar dermatosis, such as lichen sclerosus, are most commonly not associated with HPV and tend to occur in older women. A recent large-scale worldwide analysis using a homogeneous protocol for HPV testing reported HPV DNA positivity in 28.6 % of invasive vulvar tumors and 86.7 % in preneoplastic lesions. Positivity decreased to 25 % in vulvar carcinomas when in addition to viral DNA p16INK4a overexpression was also considered as a criterion for an HPV-induced cancer. HPV16 was the most common type identified representing over 75 % of all positive cases [38].
Vaginal cancer is also a rare malignancy, with an estimated of 13,000 new cases diagnosed worldwide in 2008 and accounting for about 2 % of all gynecologic cancers (Table 2) [7]. The age-adjusted incidence rates range from 0.5 to 1.7 per 100,000 women per year. Most vaginal invasive cancer cases occur in patients older than 60 years, except for adenocarcinomas, which occur in younger ages [37]. Like in vulvar cancers, the SCC is the most frequently diagnosed histological type (80–90 %), followed by adenocarcinomas. As for cervical cancer, squamous cell vaginal cancer is preceded by premalignant lesions or vaginal intraepithelial neoplasia (VAIN).
Table 16.2
Anogenital cancers associated with HPV infection and with HPV 16 and 18 types
Contribution of HPV (%) | Number of cancers | ||||
|---|---|---|---|---|---|
Site | Attributable to HPV (%) | Attributable to HPV 16/18 (%) | Total | Attributable to HPV | Attributable to HPV 16/18 |
Cervix | 99 | 70 | 530,000 | 530,000 | 371,000 |
Vulva | 43 | 37 | 27,000 | 12,000 | 10,000 |
Vagina | 70 | 61 | 13,000 | 9000 | 8000 |
Anus (female) | 88 | 79 | 15,000 | 13,000 | 12,000 |
Anus (male) | 12,000 | 11,000 | 9000 | ||
Penis | 50 | 35 | 22,000 | 11,000
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |