Anaplastic Thyroid Cancer and Miscellaneous Tumors of the Thyroid
Joshua P. Klopper
Madeleine A. Kane
Bryan R. Haugen
Introduction
The vast majority of thyroid cancers are follicular cell–derived, well-differentiated tumors categorized as differentiated thyroid cancer (DTC). From 1992 to 2006, 94% of all thyroid cancers registered in the National Cancer Institute’s SEER 13 registry database were either papillary or follicular thyroid cancers. Only 1.3% registered thyroid cancers were anaplastic thyroid cancer (ATC) and 2.2% were miscellaneous or unspecified tumor types (1). Though rare, ATC and miscellaneous tumors of the thyroid are important to properly diagnose and manage due to high morbidity, mortality, and costs for treatment, surveillance, and follow-up care.
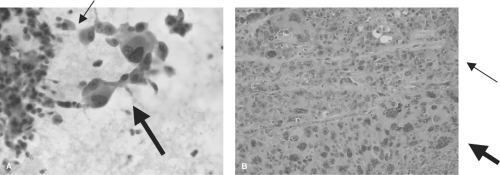 Figure 52.1 Cytopathology and histopathology of ATC. A: Cytologic specimen of ATC with epithelioid (thin arrow) and giant cell (thick arrow) morphology (Papanicolaou stain, 40×). B: Histologic specimen of ATC showing an undifferentiated tumor with both epithelioid (thin arrow) and giant cell (thick arrow) morphology (hematoxylin and eosin stain, 20×). Courtesy of Dr. Sherif Said, Department of Pathology, University of Colorado School of Medicine. See color plate. |
Anaplastic Thyroid Cancer (ATC)
Epidemiology
ATC is a very rare tumor that is associated with a very poor prognosis. The prevalence of 1.3% is less than historical
figures likely from a combination of improved definitions of ATC (separating from poorly differentiated thyroid cancer and lymphoma) and improved cytologic diagnosis (2). The majority of patients with ATC are diagnosed in the sixth and seventh decades of life (1,3). A similar incidence of ATC was noted among non-Hispanic men and women, while there was a 2.9 relative risk for women as compared to men among Hispanics. A personal history of a long-standing goiter, previous resection for PTC, and family history of other cancers were risk factors for ATC in a case–control study (3). There were no clear environmental or occupational risk factors identified.
figures likely from a combination of improved definitions of ATC (separating from poorly differentiated thyroid cancer and lymphoma) and improved cytologic diagnosis (2). The majority of patients with ATC are diagnosed in the sixth and seventh decades of life (1,3). A similar incidence of ATC was noted among non-Hispanic men and women, while there was a 2.9 relative risk for women as compared to men among Hispanics. A personal history of a long-standing goiter, previous resection for PTC, and family history of other cancers were risk factors for ATC in a case–control study (3). There were no clear environmental or occupational risk factors identified.
Etiology
The recent use of molecular diagnostics and earlier detection and treatment of poorly differentiated papillary and follicular thyroid cancers, suggests that ATC arises from a background of follicular cell–derived thyroid cancer (4). Numerous molecular mutations or events have been associated with ATC. A tissue microarray study comparing 12 ATCs to adjacent foci of DTC demonstrated a decrease in expression of the cell–cell adhesion molecule E-cadherin as well as its anchoring protein β-catenin (5). A follow-up study expanded the list of differentially expressed markers of ATC to include overexpression of MIB-1 (indicating excess proliferation) and TOPO-II (important for DNA replication and cellular proliferation amongst other pro-growth properties) when compared with adjacent foci of DTC (6). Inactivating mutation of the tumor suppressor p53 leading to protein overexpression has been identified in many ATC tumors, suggesting an important role for this tumor suppressor in progression to ATC (7,8). The well-known BRAFV600E mutation of the mitogen-activated protein kinase (MAP kinase) oncogenic signaling pathway has been described in 25% of ATCs with associated DTC, again linking the two tumor types (9). A subset of ATCs (14%) also had an increased copy number of PI3 kinase indicating over-activity of the parallel PI3K–AKT pro-proliferative pathway (9). DUSP26, a dual specificity protein phosphatase that decreases p38 mediated apoptosis, was found to be overexpressed in ATC (10). When overexpressed in ATC cells, DUSP26 caused increased proliferation, and silencing of this gene caused increased apoptosis in these cells. Other identified genetic alterations associated with ATC include upregulation of the micro-RNA miR-146a by nuclear factor-KB as well as other up- and downregulated micro-RNAs, RAS mutations, PTEN mutations, and others (11,12,13).
Diagnosis
Clinical Presentation
The typical presentation of ATC is a large anterior neck mass with rapid growth and compressive symptoms including dysphagia, dyspnea, and hoarseness due to the adjacent location of the recurrent laryngeal nerve. The invasive nature of ATC may cause hemoptysis as a presenting symptom as well. Other rare presentations include retropharyngeal masses, abdominal cutaneous metastases, and hyperthyroidism (so-called “malignant pseudothyroiditis” (14,15,16). Given the aggressive nature of ATC, distant metastases may be present at the time of diagnosis. A prospective evaluation of patients with metastatic ATC at presentation had staging PET–CT fusion studies with follow-up CT studies with contrast. In 20 patients, 35% of lesions seen on PET–CT were not visible on the other study. Additionally, 25% of patients had initial therapy altered based on the PET–CT findings (17). Therefore, patients with a neck mass and any suspicion of ATC should have PET–CT evaluation as initial staging to help optimize management decisions.
Pathologic Diagnosis
The most common histologic descriptions of ATC include spindle, giant cell, and squamoid patterns (18,19) (Fig. 52.1A). Extensive tissue necrosis due to the rapid expansion of the tumor can make cytologic diagnosis difficult, necessitating an open biopsy in some patients. A recent cytologic analysis of nine consecutive ATC patients demonstrated an 89% PPV for the preoperative diagnosis of ATC (20). If the diagnosis of ATC can be made by cytology (Fig. 52.1B), therapies such as external radiation can be started sooner than in patients requiring open biopsy.
ATC is traditionally considered a completely dedifferentiated tumor, negating the benefits of traditional thyroid molecular markers such as PAX-8 and TTF1. However, a recent analysis of ATC histopathological specimens found that 76% of tumors had expression of PAX8 by IHC (21). Thus, if the diagnosis is unclear due to the poorly differentiated nature of the tumor, PAX8 immunohistochemical analysis may be considered.
Treatment
The primary goals of any cancer therapy are to improve quality of life, prolong survival, and minimize suffering related to the cancer progression and specific therapies. Unfortunately, retrospective database and large single-center studies show that most surgical, radiation, and chemotherapy approaches do little to prolong survival in most patients with ATC, demonstrating a dismal 3- to 5-month median survival from diagnosis and a 1-year survival ranging from 9% to 23% (4,22,23,24,25,26). Rapidly assessing and choosing the right patients for aggressive (potentially curative, improved survival) versus palliative (improve quality of life, reducing suffering from aggressive therapy) intervention is critical. A recent analysis of 251 patients with ATC from the SEER database (1983–2002) showed that patients with disease confined to the thyroid, or with minimal extrathyroidal invasion, had a 2-year survival of 33% and 5-year survival of 23%, while those with gross extrathyroidal extension or distant metastases had a 2-year survival of only 2% (24). A preoperative assessment using cross-sectional imaging and PET/CT imaging can help define which patients may benefit most from aggressive versus palliative therapy (27,28). The most significant factors influencing survival of patients with ATC are distant metastases and age, while other important factors include extrathyroidal extension, tumor size, and functional status (23,24,29). Tailoring the therapy to the appropriate patient with ATC is a critical component in the management of these patients. A proposed algorithm to assess and manage these patients is presented in Figure 52.2.
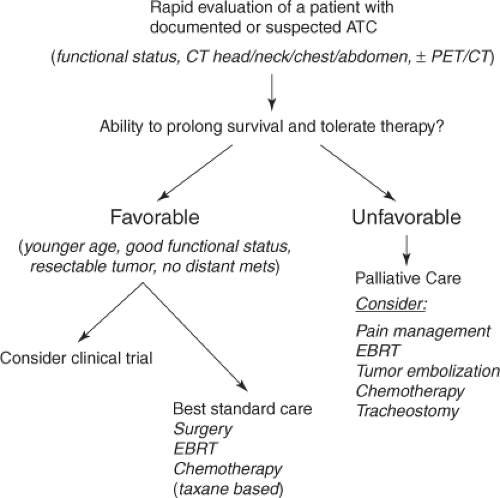 Figure 52.2 Proposed approach to rapid evaluation and treatment of patients with ATC based on “favorable” or “unfavorable” features. |
Surgery
It is difficult to assess the exact role of surgery in patients with ATC, since no study has systematically randomized the surgical approach. However, it is clear that prolonged survival is associated with a complete surgical resection of the primary tumor, which is more associated with a tumor that is intrathyroidal and can be completely resected (stage IVA), than an incomplete resection of a grossly invasive tumor (stage IVB). It is therefore important to preoperatively assess patients with careful cross-sectional imaging to determine if a complete surgical resection of the primary tumor is feasible. Sugino et al. reported a 60% 1-year survival in patients where a >90% resection was believed to be feasible based on preoperative evaluation, versus a 1-year survival of only 21% in those where total resection was not possible (30). McIver and colleagues reviewed surgical approaches over 50 years in 96 patients with ATC at the Mayo Clinic (4). They observed a trend toward improved survival with a more complete surgical resection, but this did not reach statistical significance. One-year survivals were as follows: Complete surgical resection (20%), minimal residual disease (10%), and gross residual disease (<5%). Other groups have also observed improved survival associated with a more complete resection of the primary tumor (24,25,31,32). It is not possible to determine if the better outcome in those with more aggressive therapy was due to the surgical resection itself, or the more limited disease in this group of patients, since these studies were not controlled and the groups receiving less aggressive surgical intervention generally had more advanced disease.
Based on these primarily retrospective, non-controlled surgical studies, aggressive primary tumor resection should be considered in patients without metastatic disease, where preoperative evaluation suggest that such a resection is feasible with limited morbidity. Patients with distant metastatic disease (stage IVC) and/or unresectable primary tumors should not undergo primary surgical resection and should be considered for palliative management. Large operations that include resection of the esophagus, trachea, or larynx are generally not recommended (27). A surgical approach to manage patients’ airways should be considered in those with rapidly growing primary tumors and airway distress or vocal cord paralysis (33,34).
Table 52.1A Historical Response to Therapy for Atc | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
External Beam Radiotherapy and Multimodality Therapy
External beam radiotherapy (EBRT) has been the cornerstone of both aggressive and palliative approaches to patients with ATC. A major advance in delivering radiotherapy to the thyroid bed and neck has been the use of intensity-modulated radiotherapy (IMRT), which uses computers and linear accelerators to create a three-dimensional radiation map delivering high doses of radiation to the tumor and thyroid bed while reducing exposure to normal tissue (35). Furthermore, image-guided radiotherapy (IGRT) allows for ongoing adjustments of the radiation therapy based on changing tumor volume.
Wang et al. retrospectively reviewed their experience with EBRT alone in 47 patients with ATC treated at the Princess Margaret Hospital, 1983 to 2004 (36). Twenty-three patients with locoregional disease and good performance status received aggressive radiotherapy (median 60 Gy, 45–66 Gy) and 24 patients with distant metastases or poor performance status received palliative radiotherapy (<40 Gy). Median overall survival for the entire group was 5.6 months with a 2-year survival of only 4%. Those treated aggressively had a median overall survival of 11.1 months, a 1-year survival of 46%, and 2-year survival of 9%. Six-month local progression-free rate was 94%, indicating good locoregional disease control. Many of the patients subsequently died from distant metastatic disease. This group also compared once-daily EBRT (200 cGy/fraction) with twice-daily EBRT (150 cGy/fraction started after 1997). Toxicity was similar in the two groups with only grade 3 skin toxicity. Patients receiving twice-daily EBRT had a trend toward increased overall survival (13.6 vs. 10.3 mo) and 1-year survival (67% vs. 36%), but this did not reach statistical significance, partly due to small numbers in each group. This study suggests that EBRT can provide good locoregional control with limited toxicity and that twice-daily EBRT in the appropriately selected patients may provide a survival advantage. Other groups have used “accelerated” twice-daily EBRT, which has the theoretical advantage of giving more radiation in smaller fractions over a shorter period of time (25,32,37,38). While this approach has the advantage of a shorter period of radiation treatment and may improve locoregional control, it is not clear if accelerated EBRT improves overall survival. One study noted very high grade 3 and 4 toxicity (esophagitis, mucositis, erythema), although this may have been related to very high fractions (1.8 to 2.0 cGy BID) and poor patient functional status (22% died during EBRT) (38). Careful selection of patients for aggressive therapies and newer IMRT approaches may improve outcomes and decrease toxicity in patients with ATC.
Bhatia and colleagues retrospectively reviewed the experience at MD Anderson Cancer Center for 53 patients treated for ATC from 1995 to 2007 (26). Overall median survival was 3 months with a 19% 1-year survival. Thirty-one patients had surgery and aggressive EBRT (45–60 Gy, 2–3 Gy/fraction) and 81% received concurrent chemotherapy. Interestingly, 16 of these patients had distant metastatic disease. The median overall survival in this group treated aggressively was 7 months with a 1-year survival of 29%. Five patients had no evidence of disease at last follow-up (median 44 months). None of these patients had distant metastases at presentation, but two had gross residual disease prior to EBRT. Again, this supports the approach of aggressive EBRT after surgery even in patients with gross residual disease. Toxicity was high with 23% of the patients having significant toxicity, 5 patients requiring feeding tubes, and 5 patients requiring hospitalization. Such toxicity may be unacceptable in patients with known distant metastatic disease.
Table 52.1B Recent Studies of Multimodality Therapy for Atc | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree