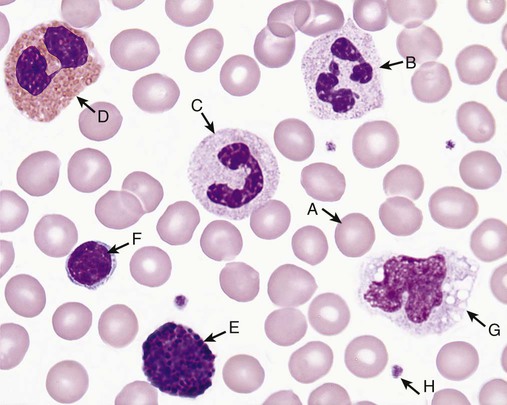
Plasma transports and nourishes blood cells. There are three families of blood cells: red blood cells (RBCs), or erythrocytes; white blood cells (WBCs), or leukocytes; and platelets, or thrombocytes.1 Hematology is the study of blood cells. By expertly staining, counting, analyzing, and recording the appearance, phenotype, and genotype of all three types of cells, the medical laboratory scientist is able to predict, detect, and diagnose blood diseases and many systemic diseases that affect blood cells. Physicians rely on hematology laboratory test results to select and monitor therapy for these disorders. Early scientists such as Athanasius Kircher in 1657 described “worms” in the blood, and Anton van Leeuwenhoek in 1674 gave an account of RBCs,2 but it was not until the late 1800s that Giulio Bizzozero described platelets as “petites plaques.”3 The development of Wright stain by James Homer Wright in 1902 opened a new world of visual blood examination through the microscope. Although many automated instruments now differentiate and enumerate blood cells, Wright’s Romanowsky-type stain (polychromatic, a mixture of acidic and basic dyes), and refinements thereof, remains the heart of blood cell identification.4 In the present-day hematology laboratory, RBC, WBC, and platelet appearance is analyzed visually using 500× to 1000× light microscopy examination of cells fixed to a glass microscope slide and stained with Wright or Wright-Giemsa stain (see Chapter 15). The scientific term for cell appearance is morphology, which encompasses cell color, size, shape, cytoplasmic inclusions, and nuclear condensation. RBCs are anucleate biconcave cells filled with a reddish protein, hemoglobin (Hb, HGB), which transports oxygen and carbon dioxide (see Chapter 10). RBCs appear pink to red and measure 6 to 8 µm in diameter with a zone of pallor covering one third of their center (Figure 1-1, A), reflecting their biconcavity (see Chapters 8 and 9). Since before 1900, physicians and medical laboratory scientists counted RBCs in measured volumes to detect anemia or polycythemia. Anemia means loss of oxygen-carrying capacity and is often reflected in a reduced RBC count (see Chapters 14 and 18). Polycythemia means an increased RBC count reflecting increased body RBC mass, a condition that leads to hyperviscosity (see Chapter 34). To count RBCs, laboratory scientists carefully pipetted a tiny aliquot of whole blood and mixed it with 0.85% (normal) saline. This saline concentration matches the osmolality of normal blood; consequently, the suspended RBCs closely retained their native morphology, neither swelling nor shrinking. A 1 : 200 dilution was typical for RBC counts, and a glass pipette designed to provide this dilution, the Thoma pipette, was used routinely until the advent of automation and is still available from clinical laboratory supply companies. The diluted blood was transferred to a counting chamber or hemacytometer (see Figure 14-1). The medical laboratory scientist observed and counted RBCs in selected areas of the hemacytometer, applied a mathematical formula based on the dilution and on the area of the hemacytometer counted (see Chapter 14), and reported the RBC count in cells per microliter (mcL), milliliter (mL, also called cubic centimeter, or cc), or liter (L). Visual RBC counting was developed before 1900 and, although never accurate, was the only way to count RBCs until 1958, when automated particle counters became available in the clinical laboratory. The first electronic counter, patented in 1953 by Joseph and Wallace Coulter of Chicago, Illinois, was used so widely that today automated cell counters are often called Coulter counters, although many high-quality competitors exist (see Chapter 39).5 The Coulter principle of direct current electrical impedance is still used for RBC counting in many automated hematology profiling instruments. Happily, the widespread availability of automated cell counters has replaced visual RBC counting. RBCs also are assayed for hemoglobin concentration and hematocrit (Hct, see Chapters 10 and 14). Hemoglobin measurement relies on a weak solution of potassium cyanide and potassium ferricyanide, called Drabkin reagent. An aliquot of whole blood is mixed with a measured volume of Drabkin reagent, hemoglobin is converted to stable cyanmethemoglobin (hemiglobincyanide), and the solution is placed in a photometer with incident light at 540 nm wavelength. The color intensity is compared with that of a reagent blank and a known standard and is mathematically converted to hemoglobin concentration. Drabkin reagent is used in manual and most automated applications, although some automated hematology profiling instruments use a formulation of the ionic surfactant (detergent) sodium dodecyl sulfate to reduce environmental cyanide. Hematocrit is the ratio of the volume of RBCs to the volume of whole blood and is determined by transferring blood to a graduated plastic tube, centrifuging, measuring the column of RBCs, and dividing by the total length of RBCs plus plasma. The normal ratio approaches 50% (see inside front cover for reference ranges). Hematocrit is also called packed cell volume (PCV), the packed cells referring to RBCs. Often one can see a light-colored layer between the RBCs and plasma. This is the buffy coat and contains WBCs and platelets. The laboratory scientist may use the three numerical results, RBC count, hemoglobin, and hematocrit, to compute the RBC indices mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC, see Chapter 14). The MCV, although a measure of volume, reflects RBC diameter on a Wright-stained blood film; the MCHC reflects RBC staining intensity or degree of pallor. The MCH expresses the mass of hemoglobin and closely reflects the MCHC. A fourth RBC index, RBC distribution width (RDW), expresses the degree of variation in RBC volume. Extreme RBC volume variability is visible on the Wright-stained blood film as variation in diameter and is called anisocytosis. The RDW is based on the standard deviation of RBC volume and is routinely reported by automated cell counters but cannot be provided using manual RBC measurements. In addition to aiding diagnosis, the RBC indices provide stable measurements for internal quality control (see Chapter 5). Laboratory scientists routinely use 1000× visual examination (see Chapter 4) to review RBC morphology, commenting consistently on RBC diameter, color or hemoglobinization, shape, and the presence of cytoplasmic inclusions (see Chapter 18). All these parameters—RBC count, hemoglobin, hematocrit, indices, and RBC morphology—are used to detect, diagnose, assess the severity of, and monitor the treatment of anemia, polycythemia, and numerous systemic conditions that affect RBCs. Automated hematology profiling instruments are used in nearly all laboratories to generate these data, although examination of the Wright-stained blood film is still essential. In the Wright-stained film, 1% to 2% of RBCs exceed the 6- to 8-µm average diameter and stain slightly blue-gray. These are polychromatophilic erythrocytes, newly released from the RBC production site, the bone marrow (see Chapters 8 and 16). Polychromatophilic erythrocytes are closely observed because they indicate bone marrow regeneration during blood loss and certain anemias (see Chapters 23 to 25). WBCs, or leukocytes, are not really blood cells; they are a loosely related grouping of cell families dedicated to protecting their host from infection and injury (see Chapters 12 and 28). WBCs “hitch a ride” in the blood from their source, usually bone marrow or lymphoid tissue, to their tissue destination. They are so named because they are nearly colorless in an unstained cell suspension. In chronic leukemia, an extreme increase in the WBC count imparts a milky appearance to the blood (see Chapters 34 and 36).
An Overview of Clinical Laboratory Hematology
History
Red Blood Cells
Hemoglobin, Hematocrit, and Red Blood Cell Indices
Reticulocytes
White Blood Cells
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Oncohema Key
Fastest Oncology & Hematology Insight Engine