Major Concepts
Diseases
Several types of clinically similar hemorrhagic fevers (HFs) due to New World arenaviruses are found in the Western Hemisphere. These are the Argentine, Bolivian, Venezuelan, Brazilian, Whitewater Arroyo virus, and Chapare virus hemorrhagic fevers, caused by the Junin, Machupo, Guanarito, Sabia, Whitewater Arroyo, and Chapare viruses, respectively. All of these diseases occur in South America, with the exception of Whitewater Arroyo virus HF.
Symptoms
All of the American HFs have a relatively high mortality rate and a low infectious dose and may be acquired by inhalation of infectious rodent excreta or person-to-person contact. Early symptoms include headache, weakness, fever, petechial and erythematous rashes, anorexia, nausea and vomiting, and lymphadenopathy. Later symptoms include bloody diarrhea, enlargement of the spleen, low blood pressure, and shock. Thrombocytopenia may cause small focal hemorrhages, and increased vascular permeability may result in hemoconcentration. Hemorrhagic manifestations are present and may be accompanied by pneumonia, neurological sequelae, deafness, dehydration, and renal, myocardial, and liver involvement. Some of the American HFs are quite rare, with only three to five reported cases, while others, such as the Argentine, Bolivian, and Venezuelan HFs, have infected hundreds to tens of thousands of individuals.
Infection
The American HF viruses belong to the Tacaribe serogroup of the Arenaviridae family and are small, enveloped, single-stranded positive-sense RNA viruses. These organisms are also called “sandy viruses” because they contain multiple particles that are similar in appearance to ribosomes, giving the viruses a sandy appearance. Almost all of the pathogenic New World arenaviruses are found in South America and belong to clade B. The Whitewater Arroyo virus and several nonpathogenic viruses are located in North America and belong to clade A. On the average, a new member of the Tacaribe serogroup is identified every three years. Several Old World arenaviruses also infect humans. These are the lymphocytic choriomeningitis virus, Lassa virus, and a currently unnamed virus found in southern Africa.
Immune Response
Levels of several cytokines increase during American HF, including IFN-α, IL-6, IL-8, IL-10, and TNF-α. IFN-α levels are particularly high in fatal cases, and this cytokine may contribute to pathology. Amounts of the hematopoietic growth factor G-CSF also rise during New World arenavirus infection, and its levels correlate with disease severity. Levels of an erythrocyte growth factor decrease, perhaps contributing to the decrease in red blood cell production. Levels of CD4+ T helper cells are reduced as well.
Treatment
Ribavirin used in combination with convalescent plasma containing virus-specific antibodies has proved effective in the treatment of Argentine, Bolivian, and Brazilian HFs. Ribavirin may also be protective in treating infection with Lassa virus and a novel Old World arenavirus found in South Africa and Zambia. Several newer drugs have been developed as well.
Prevention
Barrier precautions (gloves and masks) protect health care personnel from infection. Rodent control, such as trapping and poisoning of rodents, may reduce disease incidence in the general population. A highly effective live attenuated-virus vaccine is available to protect against infection with Junin virus. It is cross-protective against challenge with Machupo virus in animal model systems.
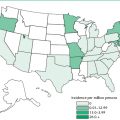
Six naturally occurring types of HFs in humans are currently recognized in the Americas: Argentine, Bolivian, Venezuelan, Brazilian, Whitewater Arroyo virus, and Chapare virus HFs. These are caused by the Junin, Machupo, Guanarito, Sabia, Whitewater Arroyo, and Chapare viruses, respectively, and belong to the Tacaribe (New World) serocomplex of the Arenaviridae family. This complex is composed of three clades: clade A consists of the pathogenic Whitewater Arroyo and the nonpathogenic Tamiami and Bear Canyon viruses, all from North America, and Pichinde, Parana, Pirital, Flexal, and Allapahuayo South American viruses; clade B contains the pathogenic Junin, Machupo, Guanarito, Sabia, and Chapare viruses and the nonpathogenic Tacaribe, Amapari, and Cupixi viruses; and clade C contains the nonpathogenic Oliveros, Latino, and Pampa viruses. However, a recent report places the three North American viruses in a distinct group. On the average, a new member of the serocomplex is found every three years. They are transmitted by sigmodontine rodents, and with the exception of the Whitewater Arroyo virus, pathogenic members are restricted to South America. The Old World arenavirus found in North America is the lymphocytic choriomeningitis virus.
RECENT DEVELOPMENTS
The cellular receptor for the Old World arenaviruses, lymphocytic choriomeningitis virus and the Lassa fever virus, and clade C but not clade A or B New World viruses is α-dytroglycan. The latter two types of New World viruses interact with human target cells by binding to the transferrin receptor, involved in iron transport into the cell. The pathogenic clade B viruses Junin, Machupo, and Guanarito all infected human and nonhuman primate cells to a high level, with lesser infection of rodent cell lines. The opposite is true of the nonpathogenic Amapari clade B member. Using a multiplicity of infection of 10, the pathogenic viruses have been found to be highly effective in establishing infection of HeLa cervical epithelial cells, A549 lung epithelial cells, Huh7 hepatoma cells, HUVEC human umbilical vein endothelial cells, and Thp-1 monocytes and less effective in infection of WIL-2 B and Jurkat T lymphocyte cell lines. Preincubation of cells with inactivated Junin or Guanarito viruses blocks infection by the other pathogenic South American arenaviruses. This is not true of the tested nonpathogenic viruses of any clade. The receptor for these viruses is sensitive to treatments that remove proteins but not phospholipids, N-linked glycosylation, α-N-galactosamine, fucose, galactose, O-linked glycosylation, or sialic acid alone. Removal of both O-glycans and sialic acid leads to a slight enhancement of infection. Neither heparin sulfate nor heparin appears to affect infection. Identification of the chemical composition of the receptors for arenaviruses may allow development of drugs that specifically block these viruses from entering cells, thus preventing infection of humans.
The American HF viruses pose unique challenges: infection with these viruses leads to a high mortality rate, vaccines against the majority of these agents are lacking, and infection may occur via inhalation. These factors, together with the low numbers of organisms required to infect a human, make these viruses good candidates for weaponization and therefore agents of special concern to the world community. Fortunately, several effective treatment options are available for use for individuals afflicted by American hemorrhagic fevers, which is not the case for some of the other hemorrhagic fevers, including one caused by infection by the Old World arenavirus Lassa fever (discussed in Chapter Fourteen).
Argentine hemorrhagic fever was first described as a clinical disease in 1955 in an epidemic in Bragado, Argentina, in Buenos Aires Province. The endemic area for this disease is continuing to spread over this province and parts of Sante Fé and Cérdoba provinces. The Junin virus, the causative agent, was subsequently isolated in 1958 in the city of Junín. Between 1958 and 1987, about 21,000 cases of Argentine HF were reported.
Scattered cases of “black typhus” began in the Bolivian Amazon River basin in 1959. The outbreak of this disease, now known as Bolivian hemorrhagic fever, resulted in 470 cases and 142 deaths (30% fatality rate) between 1959 and 1962. In the 1960s, there were large epidemics with many deaths. In one town with 3,000 residents, 600 cases occurred, with 113 deaths recorded in two years (20% incidence, 19% fatality rate). These epidemics were finally stopped by the use of mousetraps and oil-grain baits containing zinc phosphide to eliminate the rodent vector. The causative arenavirus, the Machupo virus, was first isolated in 1963. There were no cases between 1973 and 1992. A familial cluster of patients was reported in 1994.
In September 1989, an outbreak of severe febrile disease was noted in Portuguesa, Venezuela. This outbreak was confined mainly to rural inhabitants in the southern portions of the state. A total of 105 confirmed or probable cases of Venezuelan hemorrhagic fever occurred from September 1989 to May 1995. Many of the cases originated near Guanarito, in the Portuguesa state.
The first reported case of Brazilian hemorrhagic fever occurred in a 25-year-old female agricultural engineer in Sã Paulo state in 1990 and was fatal even after treatment with defoxitin and amikacin. She had worked mainly in an office. A lab worker later became infected, probably via an aerosol, was treated with intravenous fluids, and recovered. A second infected lab worker was put on intravenous ribavirin, an antiviral agent, and the fever disappeared within two days.
Flexal virus has not been reported to cause natural infection in humans yet but has resulted in moderately severe infections in two laboratory workers. It must therefore be regarded as a potential danger.
In 1999 and 2000, three cases of hemorrhagic fever caused by Whitewater Arroyo virus occurred in California. All three were fatal. The virus itself was first isolated from whitethroat woodrats in New Mexico in 1996.
The most recently discovered pathogenic member of the Tacaribe group is the Chapare virus. This clade B virus caused a cluster of infections, including one fatal case of hemorrhagic fever in a remote region of the Andes mountains of rural Bolivia in 2008. Little information about this virus is currently available.
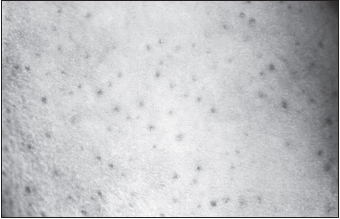
The American hemorrhagic fevers closely resemble dengue, yellow fever, idiopathic thrombocytopenic purpura, and other illness whose symptoms include prolonged fever with leukopenia and hemorrhagic signs. The prodrome (early warning signs) includes fever, sore throat, and muscle and retro-ocular pain. The infectious dose for these viruses is one to ten organisms, followed by an incubation period of 7 to 14 days. Symptoms occurring during the early stages of infection include headache, weakness, flushing of the face and thorax, petechial and erythematous rashes, anorexia, nausea and vomiting, and lymphadenopathy.
Later during the course of disease, various vascular abnormalities occur, and many patients are hospitalized. Thrombocytopenia leads to the development of small focal hemorrhages in many organs, particularly the liver (hepatocellular necrosis). The diarrhea is bloody. The number of eosinophils increases, but the total number of white blood cells decreases with both neutropenia and lymphoid depletion and necrosis in the spleen and lymph nodes. Increased vascular permeability may result in hemoconcentration. Hemorrhagic manifestations include nosebleed, bleeding gums, bloody vomit, and massive gastrointestinal hemorrhages. The spleen may enlarge and become congested. Low blood pressure may lead to shock. There is extensive infection of macrophages and, in Argentine HF, dendritic cells as well, but not lymphocytes. Lymphocyte loss in Argentine HF may be due at least in part to apoptosis. There is minimal involvement of the endothelium, with no specific vascular lesions found in infected monkeys. Substantial changes in coagulation and fibrinolysis markers may be found, including complexes of thrombin and antithrombin, prothrombin fragments, protein C, D-dimers, tissue plasminogen activator, and plasminogen activator inhibitor-1. Levels of the acute-phase proteins fibrinogen and von Willebrand factor are also increased. These changes may be caused by the presence of proinflammatory cytokines.
The nitrogen free radical nitric oxide is found in elevated amounts in the serum. This macrophage-produced mediator has both protective and deleterious properties. The latter include lymphocyte apoptosis, tissue damage, hypotension, and loss of vascular integrity, which might lead to the induction of shock. Unlike Ebola and Marburg hemorrhagic fevers, there is no disseminated intravascular coagulation.
In addition to the circulatory system, several other organ systems are affected by the American hemorrhagic fever viruses. Pneumonia may occur, with pulmonary edema and hemorrhaging. In half of a dozen fatal cases studied, secondary bacterial infections were found in the lungs. Neurological findings may include sleepiness, tremors, difficulty walking, tonic-clonic convulsions, and coma. In the case of Venezuelan HF, deafness may occur during convalescence. Other clinical findings are dehydration and renal, myocardial, and liver involvement. The renal manifestations include the presence of albumin, hyaline and granular casts, and Milani cells in the urine. Most patients recover without long-lasting sequelae after an acute illness of 10 days and a two- to three-week period of convalescence.
For patients who recover, the illness persists 10 to 14 days. Fatality rates are rather high, however. In Argentine HF, 25% to 35% of untreated cases result in death; in Bolivian HF, the figure is 30%; in Venezuelan HF, 34%; in Brazilian HF, 33%; and in hemorrhagic fever induced by the Whitewater Arroyo virus, 100%.
Argentine Hemorrhagic Fever
Argentine hemorrhagic fever preferentially strikes young male agricultural workers between the ages of 20 and 50 from the rural areas of the central-east part of Argentina, the Humid Pampa. Disease incidence is greatest in late autumn, during the corn harvest season. It is transmitted by the drylands vesper mouse (Calomys musculinus), Calomys laucha, Akodon azarae, and Mus musculus
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree