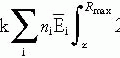Advanced Treatment Technology (IMRT, SRS, SBRT, IGRT, Proton Beam Therapy)
Intensity-modulated radiation therapy (IMRT) is capable of generating complex three-dimensional (3-D) dose distributions to conform closely to the target volume, even in tumors with concave features. With IMRT, the beam intensity (fluence) is optimized, using computer algorithms, as it is oriented around the patient (30).
This form of computer algorithm considers not only target and normal tissue dimensions but also user-defined constraints such as dose limits. This process is based on the “inverse method” of treatment planning (“inverse planning”) and is capable of generating significant dose gradients between the target volume and adjacent tissue structures to accomplish the intended dose-volume prescription (32).
Because of this specific feature, a precise mechanical system to deliver and validate the intended radiation dose to the desired area is crucial. An inverse prescription guideline that optimizes tumor target coverage and normal tissue sparing is another pertinent component of IMRT treatment.
A variety of techniques have been developed to deliver optimized IMRT. The major differences among the various approaches are in the mechanisms they use for the delivery of nonuniform fluencies.
BASIC PHYSICAL PRINCIPLES OF INTENSITY-MODULATED RADIATION THERAPY
For IMRT delivery, the fluence is to be modulated as a function of the entry angle at each point within the target volume. The dose at any point within the patient will be generated by a series of beamlets incident on that point, each with a unique entry angle.
The radiation fluence can be modulated within the cone beam using a physical modulator, a scanning dynamic multileaf collimator (MLC), a scanning bremsstrahlung photon beam, or a combination of these techniques.
Alternatively, the accelerator can be operated using dynamic motion of one or more of the angular degrees of freedom (couch, collimator, and gantry).
INVERSE TREATMENT OPTIMIZATION AND CRITERION
Reported optimization methods include (a) exhaustive search, (b) image reconstruction approaches, (c) quadratic programming, and (d) simulated annealing.
The optimization criteria in terms of dosimetric and clinical objectives are expressed as a mathematical entity in the form of an objective or cost function. The objective function defines a plan’s quality and is to be maximized or minimized, as appropriate, to satisfy a set of mathematical constraints.
Plan optimization criteria historically have been based on dose parameters. The optimization software iteratively adjusts the beam parameters to obtain the best possible results of the desired dose distribution. Recent efforts are examining the use of biologically based indices (e.g., tumor control probability and normal tissue complication probability) (10, 22).
INTENSITY-MODULATED RADIATION THERAPY TREATMENT DELIVERY SYSTEMS
Robotic pencil beam: Robot-mounted linear accelerators producing fairly narrow photon beams that move around the patient.
Rotary fan beam with intensity modulation: A commercial implementation of a fan-beam approach to IMRT, using a mini-MLC system (MIMiC) that is mounted on an unmodified linear accelerator. Treatment is delivered to a narrow slice of the patient using arc rotation (11).
The beam is collimated to a narrow slit, and beamlets are turned on and off by driving the mini-MLC leaves in and out of the beam path, respectively, as the gantry rotates around the patient. A complete treatment is accomplished by sequential delivery to adjoining slices. Helical tomotherapy, proposed by Mackie et al. (33), uses MIMiC to perform rotation IMRT while the patient is translated through the doughnut-shaped aperture in a fashion similar to the helical computed tomography (CT) scanner. In this form of tomotherapy, the problem of interslice matchlines is minimized because of the continuous helical motion of the beam around the longitudinal axis of the patient. The geometry of tomotherapy provides the opportunity to provide CT images of the body in the treatment setup position.
A system that can generate tomographic images while receiving fan-beam tomotherapy on a megavoltage linac, with the patient in the treatment position, is under development (42).
Fixed Field Multileaf Collimator
For this type of IMRT, the MLC is operated in either dynamic mode (the leaves moving while the radiation is on) or static mode, that is “step-and-shoot” mode (a sequential delivery of radiation subportals that combine to deliver the desired fluence distribution), in which the gap formed by each opposing leaf sweeps under computer control across the target volume to produce the desired fluence profile.
Intensity-Modulated Arc Therapy
This technique, developed by Yu (57), uses a combination of dynamic multileaf collimation and arc therapy. The shape of the field formed by the MLC changes continuously during gantry rotation. Multiple superimposing arcs are used and the field shape for a specific gantry angle changes from one arc to the next so that the cumulative fluence distribution is equal to the desired distribution.
Fixed Field with Compensating Filter
Filters can be designed by calculating a thickness along a ray line using an effective attenuation coefficient for the filter material. The filter construction process can be automated using numerically controlled milling machines, and generates the desired IMRT fluence profile.
Comparisons of IMRT dose distributions delivered using physical modulators and MLC-based approaches are under investigation, with indications that the dose distributions provided by these modalities are similar (49).
PATIENT IMMOBILIZATION AND IMAGE ACQUISITION
A noninvasive immobilization method is used. In the treatment of head and neck cancers, for example, the patient is placed in the supine position on a custom-made head support and a reinforced thermoplastic immobilization mask is placed around the head. This procedure allows precise repositioning over the course of treatment (31).
A volumetric computed tomography (CT) image is acquired from a dedicated CT simulator with the patient immobilized in the treatment position, and the data are transferred to an inverse planning system.
The scan slice thickness is 2.5 mm. Intended target volumes representing gross and microscopic tumor and organs at risk are defined on the prescription page of the inverse planning system.
Double-exposure portal films are obtained weekly and compared with the corresponding digitally reconstructed radiograph from initial simulation.
SPECIFICATION OF PRESCRIPTION DOSE
The desired minimum dose to target(s) and the maximum allowable dose to nontarget structures are defined in the prescription. Unlike conventional beam arrangement, the isocenter of each treatment segment may not be located within the target. Therefore, the dose is specified to each target volume.
Table 4-1 shows examples of dose prescriptions for head and neck cancer. The prescribed fraction size for the primary target is set to 1.9 Gy to increase the minimal fraction size in the lower-risk region to 1.5 Gy.
To compensate for decrease in daily fraction dose to the secondary or tertiary targets, the biologically equivalent dose (BED) was implemented, using a linear-quadratic model. An α/β ratio of 10 Gy was used to convert tumor target dose.
Based on institutional treatment guidelines for head and neck cancer, and depending on tumor volume, target doses are divided into four categories. Low-risk regions include primarily a prophylactically treated region. Intermediate-risk regions were those adjacent to the gross tumor but not directly involved by tumor. High-risk regions included the surgical bed with
soft tissue invasion by the tumor or extracapsular extension by metastatic lymph nodes (11). Gross residual disease is treated with the highest dose.
TABLE 4-1 Target Dose Specifications For Head And Neck Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
TARGET DELINEATION
Execution of IMRT requires proper knowledge of the volumes to be irradiated, based on clinical, pathologic, and radiologic information and on patterns of disease failure in addition to accurate delineation of these volumes on a 3-D basis.
DOSIMETRIC ADVANTAGE OF INTENSITY-MODULATED RADIATION THERAPY
IMRT has been shown to improve target coverage and normal tissue sparing in head and neck, cervical, prostate, and breast cancers.
Using similar dosimetric criteria to assess the merit of IMRT versus conventional beam arrangement, Cheng et al. reported that target coverage of the primary tumor was maintained and nodal coverage was improved with IMRT. Parotid gland sparing was quantified by evaluating the fractional volume of the parotid gland receiving more than 30 Gy; 66.6% ± 15%, 48.3% ± 4%, and 93% ± 10% of the parotid volume received more than 30 Gy using tomotherapy, fixed-field IMRT, and conventional therapy, respectively (p < 0.05) (12).
The emergent use of the combined-modality approach (chemotherapy and radiation therapy) for the treatment of patients with cervical cancer is associated with significant gastrointestinal and genitourinary toxicity. The unique dosimetric advantage of IMRT is the potential to deliver an adequate dose to the target structures while sparing the normal organs. It could also allow for dose escalation to grossly enlarged metastatic lymph nodes in pelvic or paraaortic areas without increasing gastrointestinal/genitourinary complications.
Portelance et al. reported that the volume of small bowel receiving the prescribed dose (45 Gy) with fixed-field dynamic MLC technique was four fields, 11.01% ± 5.67%; seven fields, 15.05% + 6.76%; and nine fields, 13.56% ± 5.30%.These were all significantly better than with two-field (35.58% + 13.84%) and four-field (34.24% + 17.82%) conventional techniques (p < .05) (38).
STEREOTACTIC IRRADIATION
Radiosurgery Techniques
For intracranial techniques, a stereotactic headframe is temporarily affixed to the patient’s skull, providing highly accurate fiducial landmarks that allow for stereotactic localization of intracranial targets after registration (i.e., image fusion) of the treatment planning CT with diagnostic neuroimaging studies, such as magnetic resonance imaging (MRI), CT, or angiography (56).
The frame provides the basis by which a target can be identified in the image study set with respect to the stereotactic frame and specified in an X-, Y-, Z-coordinate system after registration. This coordinate system is used during target localization to define the shape and extent of the lesion to be treated (55).
Stereotactic external beam radiation therapy (EBRT) differs from conventional external beam radiation therapy in several important respects.
Small volumes of 1 to 30 cm3 are treated.
When a single fraction of sterotactically guided conformal irradiation is delivered to a defined target, the term stereotactic radiosurgery (SRS) is used. The term fractionated stereotactic radiation therapy (SRT or FSRT) is used when more than one fraction is delivered over the course of several days or weeks.
Extra precision with target localization and treatment geometries is required. The best achievable mechanical accuracy is less than 0.5 mm, although a maximum error of ±1.0 mm is commonly accepted in view of the unavoidable uncertainties in target localization.
High-dose gradients at field edges minimize dose deposition outside the target volume. The volume of tissue beyond the target that receives significant dose is strongly dependent on target size and the conformity of the isodose to the target.
Beams intersect at a common point within the skull after entering through points distributed over the surface of the skull. 3-D distribution of beams reduces the volume of normal tissue receiving moderate or high doses of radiation.
INDICATIONS FOR STEREOTACTIC RADIOSURGERY
Indications for SRS include the presence of a suitably sized (generally less than 4 cm), radigraphically distinct lesion that has the potential to respond to a single, large dose of radiation.
The largest worldwide experience has been in the treatment of arteriovenous malformations (AVMs). Primary and metastatic brain tumors also have been treated.
Ideal target volumes for radiosurgery are nearly spherical and small (less than 3 cm in maximum dimension).
RADIOSURGERY SYSTEMS
A radiosurgery system consists of a stereotactic frame, a radiation delivery system, and computer hardware and treatment planning software.
Combined with the use of a conventional MRI or CT scanner, the system allows accurate determination of target size and location, treatment planning, and delivery of radiation.
Different systems that meet these requirements equally should produce equivalent outcomes in similar groups of patients.
Gamma Knife
The Gamma Knife system (Elekta AB, Stockholm, Sweden) requires a large capital purchase of approximately $3.5 million with construction of new space. The cobalt sources decay and need to be replaced, at a high cost, after 7 years.
The Gamma Knife has no other known uses beyond SRS.
The unit consists of a permanent 18,000-kg shield surrounding a hemispheric array 5,500 to 6,000 Ci of60Co, distributed in 201 sources over a portion of a hemisphere in such a way that circular beams from collimators may enter the skull through a large number of points distributed relatively uniformly over the convexity.
Four interchangeable outer collimator helmets with beam diameters of 4 to 18 mm are used to vary the target volume. Individual collimators may be plugged in to conform the dose distribution to the target shape. It produces a target size of approximately 3 to 18 mm, with a target accuracy of 0.1 mm.
The Perfexion model introduced in 2006 uses a larger patient aperture and has improved shielding.
Linac-Based System
A linear accelerator (linac) can be modified to perform stereotactic irradiation at a cost of $50,000 to $300,000, depending on whether external treatment planning devices need to be purchased.
The linac can give a target size of 10 to 50 mm, with a target accuracy of 0.1 to 1.0 mm.
Linacs have been applied for radiosurgery in various ways: (a) multiple noncoplanar arcs with circular cones; (b) noncoplanar 3-D CRT (12 to 18 fields); (c) noncoplanar 3-D conformal arc (12 to 18 fields); (d) IMRT and intensity-modulated arc therapy; (e) and CyberKnife (Accuray Inc., Sunnyvale, CA), which combines a miniaturized linac mounted on an industrial robot with a system for target tracking and beam realignment to deliver the desired dose with 6 degrees of freedom.
Conventionally, a film technique allows verification of all positioning adjustments after the coordinates of the isocenter are determined. The film technique has been gradually replaced by the modern technologies including EPID (electronic portal imaging) and OBI (on-board imaging).
TARGET VOLUME DETERMINATION AND LOCALIZATION
The technique used to determine target volume to be treated depends on the type of lesion.
Arteriovenous Malformations
The target volume should include the entire nidus of the vascular lesion, which can be visualized with radiographic angiograms, MRI, or CT.
It is important to include MRI or CT to accurately conform treatment plans with the 3-D shape of the nidus, while avoiding excessive irradiation to surrounding brain.
Neoplasms
MRI and CT are used to define treatment volumes for neoplastic lesions.
Potential limitations include errors of localization or lack of knowledge of the actual (rather than apparent) extent of the lesion, especially with infiltrative glial neoplasms.
Positron emission tomography (PET), or single-photon emission computed tomography (SPECT), is not routinely used as the primary imaging modality for stereotactic localization because of poor spatial resolution and inconsistency in determining tumor margins.
ARTERIOVENOUS MALFORMATIONS
AVMs may be symptomatic as a result of seizure disorders, vascular steal from surrounding tissue, or intracranial hemorrhage.
Hemorrhage is the most dangerous complication. AVMs bleed at a rate of 2% to 4% per year (20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree