ADRENERGIC RECEPTORS
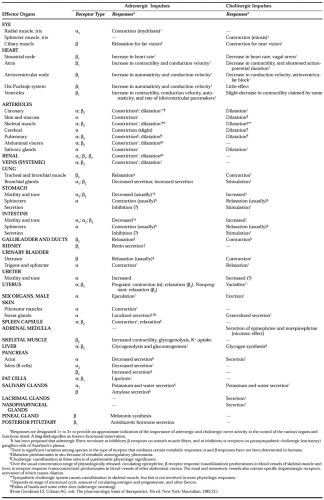
Specific receptors for catecholamines exist in several tissues, including muscle (skeletal, myocardial, vascular, gastrointestinal, ciliary, and bronchial) and glands (salivary, sweat, adrenocortical, and pancreatic).24 These receptors mediate the effects of endogenously released as well as exogenously administered catecholamines (Table 85-1).
Ahlquist25 introduced the concept of α- and β-adrenergic receptors to explain contrasting cardiovascular responses to exogenous catecholamines. NE administration causes diffuse vasoconstriction and reflexive bradycardia, whereas EPI causes vasodilation of skeletal muscle beds, tachycardia, and increased myocardial contractility. According to this framework, the vasoconstrictor actions of NE and EPI result from stimulation of α-adrenergic receptors, whereas the cardiotonic, tachycardiac, and vasodilator properties of EPI result from stimulation of β-adrenergic receptors.
Further subclassification to α1-, α2-, β1-, and β2-adrenergic receptors has been based on ligand-binding studies, on responses to synthetic agonists and antagonists, and on molecular genetic experiments.26,27 and 28 NE is an agonist at β1-, α1-, and α2–adrenergic receptors, whereas EPI is an agonist at all four subtypes. Stimulation of α1-receptors causes vasoconstriction. Phenylephrine is an example of a relatively specific α1-agonist, and prazosin is an example of an α1-antagonist. α2-Selective agonists, such as clonidine hydrochloride,29 α-methyldopa, and guanabenz,30 exert more complex effects, because stimulation of α2-receptors on vascular smooth muscle cells elicits vasoconstriction and increased blood pressure, whereas stimulation of presynaptic α2-receptors inhibits NE release from sympathetic nerve endings, and stimulation of α2-receptors in the central nervous system causes decreases in sympathetic outflow and increases in vagal outflow. The latter effects predominate, and α2-agonists are used as antihypertensive medications. α2-Antagonists (e.g., yohimbine) cause increases in plasma NE, as well as a psychiatric pattern of heightened arousal and tremulousness, in contrast with the sedation induced by α2-agonists. Pharmacologic and molecular genetic studies have demonstrated the existence of several subtypes of α2-adrenoceptors. The structure of the subtype responsible for modulation of NE release from sympathetic nerves remains unknown.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree