Chapter 76 Adrenal, Gonadal, and Thyroid Disorders
A variety of endocrine disorders have been reported in association with human immunodeficiency virus (HIV) infection and the acquired immunodeficiency syndrome (AIDS). Although direct viral infection may be responsible for organ dysfunction, most endocrine and metabolic perturbations are a consequence of systemic illness, opportunistic infections, neoplastic processes, weight loss, changes in body composition, pharmacologic therapy, or immune reconstitution. These abnormalities range from subclinical perturbations in hormone balance to overt glandular failure. While clinical presentations may bear some similarity to that in immunocompetent individuals, many aspects appear to be unique to HIV-infected patients. The goals of this chapter are to review the adrenal, gonadal, and thyroid abnormalities associated with HIV infection and AIDS and to provide a general approach to the diagnosis and management of these disorders.
ADRENAL GLAND
Adrenal Pathology
Pathologic involvement of the adrenal gland occurs frequently in AIDS, with abnormalities noted in up to two-thirds of patients during postmortem examination.1,2 However, patients are typically asymptomatic, as clinical adrenal insufficiency generally does not manifest until more than 80–90% of the adrenal gland has been destroyed.3,4 Cytomegalovirus adrenalitis appears to be the most frequent finding, although infection with M. tuberculosis and avium complex, C. neoformans and T. gondii have also been reported.1,2,5 Other pathologic findings include hemorrhage, fibrosis, infarction, and focal necrosis.5,6 Infiltration with Kaposi sarcoma or lymphoma occurs infrequently and is generally not associated with clinical adrenal insufficiency.5,7
Alterations in Adrenal Function
Individuals infected with HIV commonly demonstrate an elevation in basal cortisol levels, frequently in association with lower levels of ACTH and the adrenal steroid dehydroepiandrosterone (DHEA).8 Moreover, the adrenal reserve of the 17-deoxysteroids (corticosterone, deoxycorticosterone, 18-OH-deoxycorticosterone) appears to be impaired.9 This shift in steroid metabolism may represent an adaptive response to systemic illness10 or the effect of nonpituitary factors (e.g., cytokines) on adrenocortical function.8 Reports of increased ACTH and cortisol levels in some HIV-infected patients have led others to speculate that hypothalamic activation may occur,11 although individuals with advanced HIV illness often demonstrate blunted pituitary-adrenal responsiveness to CRH infusion.12 Increased ACTH levels may also be compensatory, particularly in patients with subclinical defects in adrenocortical function.13 In addition, glucocorticoid resistance should be considered in patients with AIDS who present with hypercortisolism, ACTH elevation, and paradoxical features of Addison’s disease.14
Pituitary-adrenal function has also been studied in the setting of HIV-associated fat redistribution, particularly in patients who present with dorsocervical fat pad enlargement and visceral adiposity. Although these features are somewhat reminiscent of Cushing syndrome, overt hypercortisolism has not been found upon biochemical examination.15–17 In addition, individuals with protease inhibitor (PI)-associated fat redistribution exhibit normal diurnal cortisol excretion as well as normal cortisol secretory dynamics after administration of ovine CRH.17 Nevertheless, subtle changes in cortisol metabolism based on urinary steroid excretion profiles have been noted in these patients, although the significance of these findings is unclear.17 An exception to the above observations has been the development of exogenous Cushing syndrome in patients treated with ritonavir in the setting of inhaled or nasal fluticasone (a glucocorticoid) where; ritonavir administration impairs the metabolism of fluticasone via effects on the cytochrome P450 3A4 enzyme system, resulting in high plasma levels of fluticasone and relative adrenal suppression.18–20
A number of drugs used to treat HIV-related disorders are known to affect adrenocortical function. For example, ketoconazole inhibits multiple steps in the pathway of cortisol biosynthesis and can cause clinical adrenal insufficiency, particularly in patients with limited adrenal reserve.21 Rifampin increases the metabolic clearance of cortisol and may also lead to diminished cortisol levels in patients with limited adrenal reserve or those receiving glucocorticoid replacement therapy.22 Megestrol acetate, a progestational agent with intrinsic glucocorticoid-like activity, has been shown to suppress the HPA axis and result in glucocorticoid deficiency when treatment is discontinued, particularly in patients who have had long-term therapy.23,24 There have also been isolated reports of Cushing syndrome and diabetes mellitus in patients receiving megestrol acetate therapy.24–26
Diagnostic Approach and Therapy
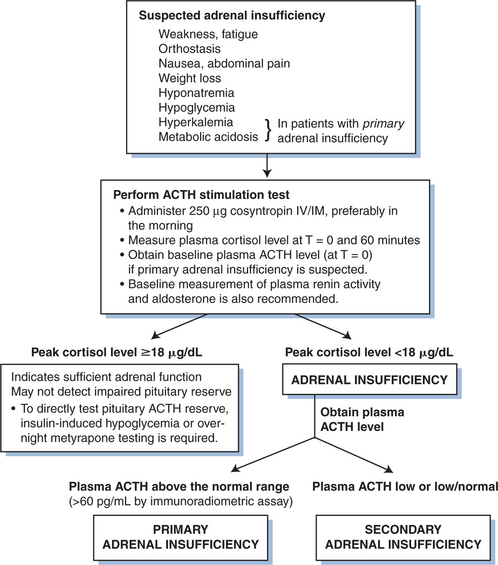
Despite the abnormalities in adrenocortical function described above, most patients are asymptomatic and clinically significant adrenal impairment is uncommon with HIV infection. However, glucocorticoid insufficiency in patients with AIDS is clearly more prevalent than in the general population, so it is important to perform tests of adrenal function in patients who present with features suggestive of adrenal insufficiency (Fig. 76-1). The signs and symptoms of adrenal insufficiency include weakness, orthostasis, nausea, abdominal pain, weight loss, hyponatremia and hypoglycemia, as well as hyperkalemia and metabolic acidosis in patients with primary adrenal failure. Hyperkalemia and renal sodium wasting have also been noted in patients receiving trimethoprim, an effect that is mediated through inhibition of the sodium channel in the distal nephron.27
The ACTH stimulation test provides the most direct means of assessing adrenal function. In patients with frank adrenal insufficiency, intravenous (or intramuscular) administration of cosyntropin (250 mcg) results in little or no increase in plasma cortisol, whereas normal individuals generally achieve peak cortisol levels above 20 mcg/dL (540 nmol/L). Basal glucocorticoid production is generally low in these patients, although the presence of low baseline cortisol levels alone should not be used for a definitive diagnosis. The ACTH stimulation test may not detect patients with impaired pituitary reserve, however, and in selected cases, insulin-induced hypoglycemia or the metyrapone test may ultimately be warranted to assess the HPA axis.28
Once the diagnosis of adrenal insufficiency has been established, it is important to distinguish between primary and secondary adrenal failure. Individuals with primary adrenal failure (Addison’s disease) invariably have high ACTH levels, and biochemical findings (hyperkalemia, metabolic acidosis) are generally consistent with concomitant mineralocorticoid deficiency. Aldosterone levels are low despite increased plasma renin levels. Adrenal computed tomography (CT) imaging may yield information about the etiology of adrenal disease in some patients,29 although in many cases the findings are nondiagnostic. Conversely, ACTH levels are low to low normal (<20 pg/mL by immunoradiometric assay) in patients with secondary hypoadrenalism due to pituitary ACTH deficiency. Such patients have normal levels of plasma renin and aldosterone.
Patients with documented adrenal insufficiency, based on failure to respond to stimulation with ACTH, should be treated with glucocorticoid replacement therapy. Hydrocortisone (20–30 mg/day) is generally administered in two divided doses, with higher doses in the morning to simulate normal circadian rhythmicity. In addition, fludrocortisone (0.05–0.10 mg/day) is usually added for mineralocorticoid replacement therapy in patients with primary adrenal insufficiency, although many patients with Addison’s disease can be managed with cortisol and adequate dietary sodium intake alone. Larger doses of glucocorticoid are required for periods of stress, generally a two- to threefold increase for moderate stress and maximal stress doses (hydrocortisone 100 mg every 8 h) for severe illness or trauma. The role of glucocorticoid therapy in patients with elevated basal cortisol levels who show somewhat diminished responsiveness to ACTH is less clear, however, and each case should be evaluated individually. A number of these patients respond to prolonged (72 h) ACTH infusion,9 suggesting that patients with a “borderline” response to acute ACTH stimulation may not require routine glucocorticoid maintenance therapy. However, some clinicians would give glucocorticoids to such patients at times of stress, provided the treatment is limited in duration, thereby avoiding the adverse consequences of prolonged steroid therapy. Routine glucocorticoid supplementation in patients with modest perturbations in the HPA axis is probably not warranted.
Special consideration should also be given to patients terminating pharmacologic glucocorticoid or megestrol acetate therapy because long-term treatment with these agents can lead to secondary adrenal insufficiency.23,24 Although adrenal function generally recovers, the process can take months; and patients with subnormal ACTH stimulation test results may have to be maintained on low glucocorticoid replacement doses until a normal cortisol response is achieved.30
DHEA and HIV Infection
DHEA is a weak androgen produced by the adrenal gland. Although present in highest concentration during early adulthood, levels decline with advancing age and chronic illness, in contrast to cortisol levels, which remain relatively stable.31,32 It has been postulated that the decline in DHEA levels may be responsible, in part, for the various immunologic, cognitive, and body composition changes associated with the aging process, leading to the widespread use of DHEA for its purported “fountain of youth” properties.31 Furthermore, a number of preliminary studies support the role of DHEA as a potential immunomodulatory agent,33–36 including pilot studies demonstrating inhibition of HIV replication in vitro,37,38 and have led to renewed interest in DHEA therapy in the HIV community.39 However, a recent small randomized trial did not show any benefit of DHEA on antiviral, immunomodulatory or hormonal effects.40 Rather, nonsignificant increases in titers of infectious HIV culturable from blood were seen in the DHEA arm and thus it was recommended that DHEA be used with caution.40 This same study did show an improvement in quality of life with DHEA administration. Higher dose DHEA supplementation has also been shown to improve mood in HIV-infected men with mild depression and significantly increase circulating sex steroid levels.41,42
TESTES AND OVARIES
Testicular Pathology
Examination of the testes at autopsy in men with AIDS have demonstrated a number of histopathologic changes, including hypospermatogenesis, basement membrane thickening, interstitial fibrosis, and tubular atrophy.43–46 Multiple factors contribute to these findings, such as prolonged HIV illness, direct HIV cytopathic effects, chronic infection, fever, malnutrition, wasting, and the use of gonadotoxic and antiandrogenic drugs. Fewer than one-third of patients demonstrate specific testicular infection.43,44 Direct testicular involvement by opportunistic infection has been seen most commonly with cytomegalovirus, T. gondii, and Mycobacterium avium complex.43,44,46 Neoplastic processes affecting the testes occur infrequently, although Kaposi sarcoma and lymphoma of the testes have been observed in patients with disseminated disease.7,46 Changes in semen quality have been reported in men with advanced HIV infection, including decreased sperm count and motility, reduced semen volume, increased abnormal sperm forms, and pyosemia.47–49
Alterations in Testicular Function
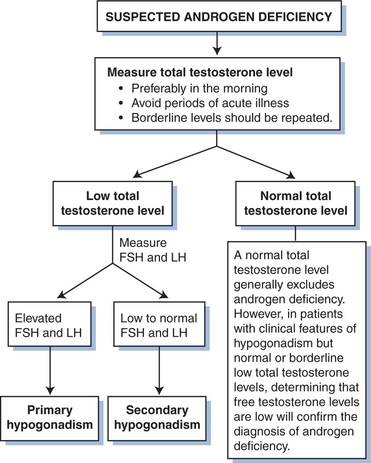
In normal individuals, gonadotropin-releasing hormone (GnRH) synthesized in the hypothalamus stimulates release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary gland. LH, in turn, stimulates production of testosterone by testicular Leydig cells, and FSH promotes spermatogenesis in Sertoli cells. Both FSH and LH secretion are regulated by androgen concentrations; LH levels reflect testosterone feedback, and FSH levels are predominantly regulated by inhibin produced by the Sertoli cells. Early in the course of HIV infection, total and free testosterone levels may be normal or slightly elevated;50,51 an exaggerated LH response to infusion of GnRH has been observed in a subset of patients.51 However, testosterone levels tend to fall with advanced HIV illness, generally as a consequence of gonadal and extragonadal factors that lead to testicular dysfunction.50,52,53 At that point, bioavailable or free testosterone levels may be the most sensitive indicator of hypogonadism, as sex hormone-binding globulin levels can be elevated in the setting of HIV infection52,54,55 (Fig. 76-2).
With primary gonadal failure, FSH or LH levels (or both) are increased in the presence of decreased androgen production. Contributing factors include direct viral or opportunistic infection, prolonged fever, malignant infiltration, and administration of gonadotoxic agents, although the underlying cause is often not identified.53,56 Ketoconazole inhibits steroidogenesis in the adrenal gland and testes, and prolonged treatment can lead to primary hypogonadism and gynecomastia, particularly at high doses.21 The majority of HIV-infected patients with low circulating testosterone levels have secondary or central hypogonadism, which is established biochemically by detecting low or inappropriately normal serum FSH and LH concentrations in the presence of low testosterone levels. In most cases the etiology is not known but is likely multifactorial, as systemic illness, malnutrition, and wasting are common causes of central gonadotropin suppression.7,53 A significant number of patients with the AIDS wasting syndrome have some degree of testosterone deficiency, and it has been suggested that the decline in circulating androgen levels may contribute to the critical loss of lean body mass.54,57 In addition, systemic administration of glucocorticoids, megestrol acetate, and opiate drugs may also contribute to central hypogonadism,58–60 as can previous treatment with anabolic androgenic steroids in cases in which recovery of the pituitary-gonadal axis is delayed. Direct pituitary destruction by opportunistic pathogens or malignant processes occurs rarely.61,62
The emergence of gynecomastia in the setting of antiretroviral therapy (e.g., protease inhibitors (PIs), efavirenz or stavudine)63–66 has additionally prompted consideration that HIV-specific treatment may alter the sex steroid bioavailability or action, since benign gynecomastia has traditionally been associated with a lower ratio of testosterone to estrogen. A number of these cases have been associated with the fat redistribution syndrome, suggesting a potential underlying metabolic component,65 although it is important that pseudogynecomastia due to increased fatty tissue accumulation (lipomastia) in the breast be distinguished.64 Other possible contributing factors to the development of gynecomastia in HIV-infected men include chronic alcoholism, marijuana or opiate drug use, liver disease, and use of medications known to cause gynecomastia. In the clinical setting, however, the most frequent cause of gynecomastia in an HIV-infected man will likely be hypogonadism.67
Diagnostic Approach and Therapy
Androgen replacement therapy is indicated in patients with symptomatic hypogonadism. Although bioavailable testosterone levels are often subnormal and not extremely low, most individuals respond to replacement therapy with symptomatic improvement. Currently, there are three main routes of testosterone delivery. The testosterone esters enanthate and cypionate have traditionally provided a safe, effective, inexpensive approach to androgen replacement therapy. For men, starting doses range from 150 to 200 mg administered by intramuscular injection every 2 weeks68 (or 300 mg every 3 weeks) although dosages may have to be adjusted for a clinical response. Because interval injections lead to large fluctuations in serum testosterone levels, some patients prefer treatment with smaller, more frequent doses (e.g., 100 mg/week). The transdermal testosterone delivery systems (patch or gel) provide a more stable, continuous mode of testosterone administration and may be preferred in patients who experience disturbing fluctuations in energy level and sexual function, although the disadvantage is that they require daily application. The testosterone patch can be associated with a local skin rash. Care should be taken with use of testosterone gel since vigorous skin contact in the applied area can lead to significant drug transfer, as residual testosterone remains on the skin after application.68 It should also be noted that the transdermal testosterone formulations may not deliver an adequate testosterone concentration at the recommended doses in every individual, so measurements to assess whether therapeutic levels are achieved should be considered. Testosterone replacement is contraindicated in men with known breast or prostate malignancies; and prostate-specific antigen (PSA) levels should be measured in older patients before and at least 3 months after testosterone treatment.68,69
Sexual dysfunction is prevalent among men with HIV infection, particularly in the setting of advanced HIV disease.70 However, not all HIV-infected men are hypogonadal, because diminished libido and erectile dysfunction may be related to a variety of other factors, such as neurologic disease, vascular causes, systemic illness, medications, substance abuse and psychosexual issues.70–73 Erectile dysfunction remains prevalent in the current era of potent combination antiretroviral therapy,73,74 potentially attributable to the aging male HIV population or, as suggested in some case series, the effect of PI therapy.73,75–78
In many patients, phosphodiesterase 5 (PDE5) inhibitors have been used successfully to improve erectile function (in the absence of other treatable causes such as hypogonadism). However, clinicians should be aware that the three available PDE5 inhibitors (sildenafil, vardenafil and tadalafil) are primarily metabolized by cytochrome P450 3A4, the hepatic microsomal enzyme involved in the metabolism of various PIs (e.g., saquinavir, ritonavir, indinavir, nelfinavir).79,80 Hence, co-administration of PIs with PDE5 inhibitors should be done with caution at the recommended low doses to avoid substantial elevation in PDE5 inhibitor concentration, particularly with indinavir and ritonavir, and risk of PDE5 inhibitor-related adverse events.79,80
Hypogonadism with decreased testosterone levels has been reported in HIV-infected women as well, although the true incidence of this disorder in women is poorly characterized. The approach to diagnosis is the same as in men, however replacement doses of testosterone should be ∼300 mg/day, preferably delivered via a transdermal patch.81,82
Ovarian Pathology and Function
Because the ovaries have not been systematically examined during autopsy studies in women with AIDS, little is known regarding ovarian pathology in the setting of HIV infection. Thus far there has been rare reports of cytomegalovirus (CMV) oophoritis in women with CMV infection.83,84 HIV infectivity has also been demonstrated in tissues of the female reproductive tract,85 and the presence of HIV RNA has also been detected in follicular fluids and cumulous oophorus cells in HIV-infected women undergoing in vitro fertilization.86
Changes in menstrual function among women with HIV infection have been more carefully evaluated since the early 1990s. It has been suggested that rates of menstrual disturbances are increased in HIV-infected women without AIDS-defining illnesses,87 although other studies indicate that infection with HIV and related immunosuppression do not have clinically significant effects on either menstruation or vaginal bleeding.88,89 Data from two large cohorts also demonstrated that HIV serostatus per se has little overall effect on menstrual cycle length, variability, and rates of amenorrhea; however, among HIV-infected women, low CD4+ T-lymphocyte counts and high HIV viral loads were associated with polymenorrhea and increased cycle length.90 The prevalence of amenorrhea is increased in women with AIDS wasting compared to women with AIDS who are weight-stable or manifest only mild weight loss,91 findings that are not unexpected for women with a severe catabolic illness complicating their HIV infection. In addition, the use of narcotics, marijuana, and chronic alcohol consumption may similarly affect menstrual status and ovulation.59 Among HIV positive women who do report regular menstrual cycles, normal circulating levels of the ovarian hormones estradiol and progesterone have been observed during the follicular and luteal phases.92
During the era of highly active antiretroviral therapy (HAART), various body shape changes, including breast enlargement, abdominal obesity, and wasting of fat in the extremities and gluteal region, have been observed in women with HIV infection.93–95 These alterations in fat distribution have generally occurred in the absence of overt endocrine perturbations, except for rare cases associated with polycystic ovary syndrome96 or tumor-level serum testosterone concentration.97 While a preliminary study found that women with HIV-associated fat redistribution have higher concentration of androgens, insulin and LH/FSH, compared to HIV-infected women without fat redistribution and seronegative controls98 most HIV-infected women do not have features of polycystic ovary syndrome despite significant hyperinsulinemia and abdominal fat accumulation.99
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree