(1)
Breast Unit-Medical Oncology, Sint-Augustinus Cancer Centre, Iridium Network, Antwerp, Belgium
(2)
Medical Oncology, EUSOMA Breast Unit, Sint-Augustinus, Oosterveldlaan 24, Wilrijk, Antwerp, Belgium
Keywords
AdjuvantBiological targetingTrastuzumabHer-2PertuzumabLapatinib37.1 Introduction
Adjuvant therapies (radiotherapy, chemotherapy, endocrine and targeted agents) in patients with localised cancer are aimed at the elimination of putatively present micrometastatic disease. The underlying concept being that this minimal amount of disease is more amenable to definitive eradication as compared to macroscopic disease. The history of adjuvant therapy in breast cancer originated with the introduction of combination chemotherapy with CMF (cyclophosphamide, methotrexate, 5-fluorouracil) in the 1970s by the Milan group [1]. Initially this was introduced for patients with node-positive disease. Subsequently its benefit was also demonstrated in unselected patients with breast cancer without nodal involvement. Although the use of anthracyclines was also pioneered by the same Milan group, the introduction of adjuvant Adriamycin was established by the NSABP group [2]. These developments were paralleled by the use of adjuvant endocrine interventions. These initially included the use of oophorectomy and tamoxifen [3, 4]. It took until the adjuvant meta-analysis in 1998 by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) to definitively make the point that tamoxifen was only effective in patients with oestrogen receptor (ER)-positive breast cancer [4]. Criteria such as tumour (T)-stage, the presence of nodal disease, age, menopausal status and ER expression were henceforth used to tailor adjuvant therapy.
The reproducible identification of intrinsic breast cancer subtypes by genome-wide expression profiling studies and their validated prognostic significance led to gradual changes in adjuvant therapy guidelines [5–8]. Most notably, the conversion of these expression-based intrinsic subtypes into clinically defined subtypes (albeit an imperfect translation), based on routine immunohistochemical methods, modulated the selection of different adjuvant options according to these subtypes. The availability of multigene tests, including both prognostic and predictive assays, will further tailor and in general limit the use of adjuvant chemotherapy in patients with ER-positive disease and potentially might enable selective extension of the duration of endocrine agents in characterised subgroups [9–11].
37.2 Endocrine Treatment
Current adjuvant endocrine regimens and their indications are discussed separately (► Chap. 35). In summary, tamoxifen for at least 5 years and for up to 10 years remains the standard of care for all premenopausal patients with ER and/or progesterone receptor (PgR)-positive breast cancer [12]. The combination of ovarian suppression and tamoxifen or an aromatase inhibitor (AI) should be strongly considered in those premenopausal patients with high-risk disease [13]. In postmenopausal patients with endocrine sensitive disease, 5 years of an aromatase inhibitor (AI) reduces 10-year breast cancer mortality rates by 15% compared with 5 years of tamoxifen [14]. Even longer continued use of an AI for up to 10 years even after a preceding 5 years of tamoxifen is effective in increasing further disease-free survival [14].
37.3 Vascular Endothelial Growth Factor (VEGF)-Directed Treatment
Extensive preclinical work has established angiogenesis, the sprouting of new blood vessels originating from a preexisting vasculature, as one of the hallmarks of cancer [15–17]. Understanding of these pathways has progressed since the early days of angiogenesis research. Angiogenesis was once suggested to be a universal prerequisite for any tumour growth and thus a potentially universal target in patients with growing cancers. It has now been shown to be more relevant in some tumours than in others. Most angiogenesis inhibitors target, or at least co-target, the VEGF-VEGF receptor type 2 (VEGF-R2) pathway. The efficacy of VEGF-directed therapy is clearly successful in increasing overall survival (OS) in patients with advanced clear cell renal cancer and metastatic colorectal cancer, either as a single agent or in combination with chemotherapy, respectively. However, in both these diseases, anti-VEGF strategies have failed to demonstrate any benefit in the adjuvant setting. In metastatic breast cancer, the addition of bevacizumab to different types of chemotherapy, used in first- or later lines of treatment, resulted in significant gains in response rate (RR): in significant but modest gains in progression-free survival (PFS) but without a convincing influence on overall survival (OS) [18, 19]. Similarly, in the neoadjuvant setting, the addition of bevacizumab to standard chemotherapy in general results in an increase in the rate of pathological complete response (pCR), although data are somewhat conflicting. Different studies have investigated the efficacy of adding bevacizumab to standard adjuvant therapy, according to clinically defined breast cancer subtypes. The use of an angiogenesis inhibitor in the adjuvant setting has a number of clear rationales: preclinical synergy with different cytotoxics (notably with taxanes), synergy with anti-HER2 agents, the increase in response rates in metastatic disease, the immunomodulating properties of VEGF and the concept of angiogenesis inhibition as means to induce and/or sustain tumour dormancy. Three large randomised phase III studies investigated the adjuvant value of bevacizumab in the three different breast cancer subgroups.
In the largest study ever in triple-negative breast cancer, the Beatrice trial, 1290 patients were randomised to receive chemotherapy alone and 1301 to receive bevacizumab plus chemotherapy [20]. Most patients received an anthracycline-containing therapy, and 63% had node-negative disease. The 3-year invasive disease-free survival (IDFS) was 82·7% with chemotherapy alone and 83·7% with the combination. No difference in OS was observed.
In the Eastern Cooperative Oncology Group E5103 study, 4994 patients with HER2-negative breast cancer were assigned to one of three treatment arms [21]. In addition to doxorubicin and cyclophosphamide followed by weekly paclitaxel, patients received either placebo (Arm A), or bevacizumab during chemo (Arm B) or bevacizumab during chemo followed by bevacizumab monotherapy for ten cycles (Arm C). The primary endpoint was invasive disease-free survival (IDFS). Nearly two out of three patients had ER+ disease (64%). With a median follow-up of 47.5 months, the 5-yr IDFS (95%CI) was not different between these three regimens, respectively, 77% (70.9%–81.2%), 76% (71.5%–79.8%) and 80% (77%–82.5%).
Finally, in the BETH study, 3509 women with HER2-positive breast cancer who were either node-positive or high-risk node-negative (41%) were included [22]. Patients were enrolled in one of two chemotherapy regimens: six cycles of docetaxel/carboplatin plus trastuzumab (TCH) with or without bevacizumab or an anthracycline-based regimen involving three cycles of docetaxel plus trastuzumab given with or without bevacizumab followed by three cycles of 5-fluorouracil, epirubicin and cyclophosphamide (FEC). In both regimens, patients continued trastuzumab with or without bevacizumab after chemotherapy to complete 1 year of targeted therapy. Bevacizumab added to adjuvant chemotherapy plus trastuzumab had no effect on IDFS. At a median follow-up of 38 months, IDFS rates were 92% for both groups of the TCH cohort. A secondary endpoint compared IDFS in patients in the anthracycline-based vs the TCH-based cohorts and also found no significant differences between the regimens, whether with or without bevacizumab.
These studies have ended the discussion on a possible role for bevacizumab in the adjuvant setting in patients with breast cancer.
37.4 HER2-Directed Treatment
The HER2-positive subgroup of breast cancer is defined by increased expression and/or amplification of the HER2 gene. Consensus criteria of what is to be considered HER2-positive disease is defined by the most recent American Society of Clinical Oncology/College of American Pathologists (ASCO-CAP) guidelines [23]. These criteria have changed somewhat over time. Some discussion remains regarding borderline cases and heterogeneity. In general, some 15–20% of patients presenting with primary localised breast cancer belong to this subgroup. Some 50–60% of these patients also express the ER and/or the PgR in their tumours.
The introduction of trastuzumab combined with chemotherapy in first-line treatment of HER2-positive metastatic breast cancer resulted in a clinically significant survival benefit. This gain in OS was observed with the combination of trastuzumab with either anthracyclines or paclitaxel [24]. This impressive improvement was however accompanied by the emergence of cardiac toxic effects of trastuzumab, most notably if used in combination with anthracyclines.
Different randomised trials have investigated the benefit of at least 1 year of trastuzumab combined with standard adjuvant chemotherapy. Positive interim analyses were first reported in 2005 [25, 26]. Other studies have investigated different durations of trastuzumab and the combined neoadjuvant and adjuvant use of trastuzumab.
The HERA trial and the NCCTG N9831 studies used sequential trastuzumab in at least one study arm, starting the antibody after the end of chemotherapy [25, 26]. The BCIRG 006 trial asked the question of the efficacy of a non-anthracycline/trastuzumab combination as one of the experimental arms [27].
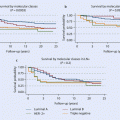
In the initial analysis, outcomes were reported with median follow-up of between 24 and 36 months. The range of benefit in DFS in favour of trastuzumab resulted in hazard ratios (HR) of between 0.48 and 0.67 (p < 0.0001), and the range in benefit in OS was between 0.59 and 0.67 (p = NS to p = 0.015). Absolute improvements in DFS ranged from 6% to 11%. With longer follow-up of these trials (8-year median follow-up from HERA and from the combined analyses of NSABP B-31 and NCCTG N9831 and 10 years for BCIRG 006), there continues to be statistically and clinically significant improvements in DFS and OS [28–30]. The magnitude of benefit appears to have somewhat decreased over time as more events (both relapses and deaths) occur, but absolute gains in OS are larger now than in earlier analyses. The selective crossover of some of the patients initially randomly assigned to the no trastuzumab arm will have mitigated some of the initial differences. However, relapses unfortunately continue to occur at a relatively constant rate over time in the trastuzumab-treated arms. Overall, in the combined NSABP B-31 and NCCTG N9831 data, an estimated improvement in 10-year DFS of 40% (HR 0.60, CI 0.53 to 0.68, p <.001) and an increase of 10-year OS from 75.2% to 84% (HR0.63, CI 0.54 to 0.73, p < .001) has been shown [30]. These improvements were observed in all subgroups, suggesting a benefit irrespective of tumour size, hormone receptor status, nodal status or patient age. Similarly for the HERA trial, with a median follow-up of 8 years, and despite a crossover in half of the patients, a significant improvement in DFS and OS was observed with HRs of 0.76 for both DFS and OS.
37.4.1 Concurrent or Sequential HER2 Blockade
The analysis of the sequential versus concurrent trastuzumab arms of the in NCCTG N9831 trial suggested a positive risk to benefit ratio for the concurrent paclitaxel and trastuzumab regimen. This resulted in the concurrent regimen as being the standard of care treatment [31].
37.4.2 Role of Dual Adjuvant HER2 Blockade
The first attempt to escalate the degree of HER2 inhibition consisted in the implementation of dual HER2 blockade by the addition of lapatinib (a reversible tyrosine kinase inhibitor of HER1 and HER2) to trastuzumab. This attempt was first tested in the neoadjuvant setting in the NeoALTTO trial. The dual blockade of HER2 translated into a near doubling of the pathological complete response rate (pCR) [32]. Unfortunately, in the four-arm adjuvant ALTTO trial, the arm with the dual HER2 inhibition failed to improve on the standard trastuzumab arm [33]. Adding lapatinib to adjuvant trastuzumab either in sequence or in combination failed to improve DFS but added toxicity. There has been much discussion about why the results of NeoALTTO failed to translate into clinical benefit in the ALTTO trial. But similar findings were made in the NSABP B-41 randomised trial of neoadjuvant therapy in operable, HER2-positive breast cancer where the addition of lapatinib, whilst increasing the pCR rate, failed to make a difference to DFS or OS [34].
Following the impressive results of dual HER2 inhibition with trastuzumab and pertuzumab in the Cleopatra study, clinical assessment of the adjuvant performance of the combined trastuzumab/pertuzumab regimen is ongoing in the APHINITY trial, which compares trastuzumab/pertuzumab and chemotherapy vs trastuzumab and chemotherapy for adjuvant treatment. At present there is no role for dual HER2 inhibition in the adjuvant setting.
37.4.3 Role of Dual Adjuvant VEGF/HER2 Inhibition
In spite of a sound preclinical rationale, negative results were reported for the addition of bevacizumab to adjuvant trastuzumab treatment within the context of the BETH study [22].
37.4.4 Role of Extended HER2 Blockade
The HERA trial was a three-arm study comparing observation to 1 or 2 years of adjuvant trastuzumab after the end of standard chemotherapy. In the comparison between 1 and 2 years of consecutive trastuzumab, the hazard ratio between both arms was 0.99 (0.85 to 1.14, p = 0.86) [35]. Although this study was also negative for both the ER+ and the ER- subgroups, there was a temporary and not statistically significant separation of the curves in favour of 2 years of trastuzumab in the ER-negative cohort, which is indicative of an increased short-term risk of relapse in the control group, who did not receive any adjuvant therapy during that time. The conclusion of the HERA extension arm remains that increasing the duration of trastuzumab beyond 1 year after chemotherapy is not an effective strategy.
In the ExteNET study, 2840 patients, who had completed up to 2 years of the standard therapy of 1 year of adjuvant trastuzumab, were randomised between observation and daily intake of 240 mg of neratinib, on oral irreversible HER1, HER2, and HER4 inhibitors [36]. The patient population enrolled in ExteNET had a higher risk of recurrence compared with the HERA patients. Neratinib is an oral agent with significant activity in the metastatic setting both in trastuzumab pretreated and unpretreated patients [37]. Its main toxicity is diarrhoea. Grade 3 diarrhoea occurred in 40% of the patients in the neratinib arm of ExteNET. This led to dose reductions in 26% of patients and drug discontinuation in 17%. An intensive loperamide prophylaxis schedule has since been shown to decrease the incidence of grade 3 diarrhoea to below 20%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree