Adjuvant chemotherapy is effective in reducing the risk of recurrence and death from breast cancer. Tumor stage and biologic characteristics are important when making decisions about who should receive chemotherapy and what chemotherapy to give. The results of ongoing clinical trials will establish whether more precise determination of prognosis and populations most likely and least likely to benefit from specific therapies can improve the efficacy and reduce the toxicity of systemic treatments. As individual tumors are molecularly characterized and molecularly targeted therapies are clinically validated, “personalized” adjuvant therapy will become a reality in the not too distant future.
Breast cancer mortality has been falling in the United States over the last several decades. This trend has been attributed to early detection as a result of the widespread adoption of screening mammography and the use of effective adjuvant therapies. Nevertheless, despite recent advances breast cancer remains a considerable public health issue. Globally it is estimated that 1.5 million women will be diagnosed with breast cancer in 2010. In the United States, 192,370 new breast cancer diagnoses (comprising 27% of all incident cancers in females) and 40,000 breast cancer deaths were estimated for 2009. Better adjuvant therapies are needed to reduce the burden of illness caused by breast cancer.
Adjuvant chemotherapy is directed at treating occult micrometastatic disease, thereby reducing the risk of recurrence. Chemotherapy works most effectively when the tumor volume is small and still in the linear growth phase. This rationale led to some of the first adjuvant clinical trials of polychemotherapy versus observation in lymph node–positive breast cancer that showed improved disease-free survival (DFS) and overall survival (OS). Subsequently in 1988, based on the early results of several randomized trials evaluating systemic therapy in lymph node–negative breast cancer, the US National Cancer Institute (NCI) issued a “clinical alert” that resulted in the recommendation by the 2000 National Institutes of Health (NIH) Consensus Conference that chemotherapy should be considered in all women with tumors greater than 1 cm or positive lymph nodes.
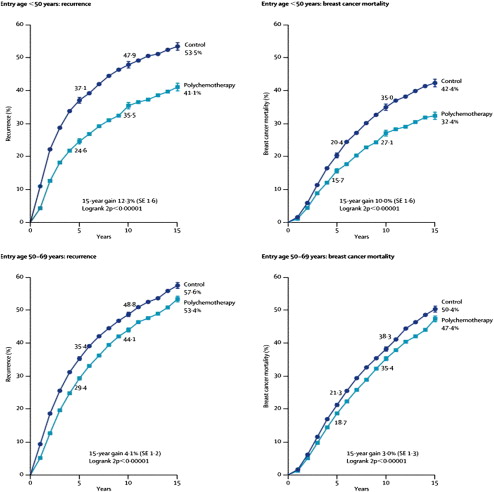
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analyses (Oxford Overview, Overview Analyses ) have provided extensive evidence illustrating the efficacy of adjuvant chemotherapy in early breast cancer. The EBCTCG was established in 1984 to 1985 and conducts 5-yearly worldwide meta-analyses of centrally collected data from every individual patient enrolled in all randomized trials already running for at least 5 years. The most recent publication in 2005 reports the 2000 meta-analyses and was based on data from 60 randomized trials initiated before 1995 comparing polychemotherapy to none, and included 29,000 women of whom 10,000 died. At 15 years there was a significant reduction in the absolute risk of recurrence and breast cancer mortality for polychemotherapy compared with none ( Fig. 1 ). The absolute benefits at 10 or 15 years were 3 times greater for younger compared with older women. In younger women adjuvant polychemotherapy reduced the annual risk of relapse and death by 37% and 30%, respectively, and the absolute gain in survival was twice as great at 15 years as it was at 5 years (10% vs 4.7%). In older women (50–69 years) the annual risk of relapse and breast cancer mortality was reduced by 19% and 12%, respectively, and translated into an absolute gain of 4.1% and 3.0%, respectively, at 15 years. The proportional reductions in recurrence and breast cancer mortality were similar in node-negative and node-positive patients. However, the absolute benefit was greater in node-positive patients. The Overview Analyses also indicate that the greatest effect of adjuvant chemotherapy occurs in the initial few years after therapy; however, the effects last for long periods of time, exceeding 15 to 20 years.
Further subgroup analysis showed that adjuvant chemotherapy was effective in both estrogen receptor (ER)-negative and ER-positive breast cancer. However, the Overview Analyses and data from several Cancer and Leukemia Group B (CALGB) trials in patients with node-positive breast cancer indicate greater chemotherapy benefit in ER-negative compared with ER-positive disease. The first report from the 2005-2006 Overview Analyses specifically considered the benefit of chemotherapy in ER-negative tumors. In this analysis there were 20,000 women with ER-negative breast cancer randomized to polychemotherapy versus none. Significant reductions were observed in the absolute risk of recurrence and cancer-specific mortality of 12.3% and 9.2%, and 8.6% and 6.1% for patients younger than 50 years and between 50 and 69 years, respectively. These data are based on clinical trials conducted with older regimens, and it is expected the proportional reductions observed for recurrence and mortality would be greater with contemporary chemotherapy.
Decisions regarding adjuvant chemotherapy have been based predominately on anatomic stage (tumor size [T], the status of the surrounding lymph nodes [N], and the presence of distant metastases [M]). The American Joint Committee on Cancer (AJCC) developed the TNM classification in 1959 when less was known about the influence of tumor biology on prognosis. Fifty years later the TNM classification continues to provide essential prognostic information; however, there has been a conceptual shift. Breast cancer is no longer considered as a single disease entity but rather a group of molecularly distinct subtypes each with different prognoses, natural histories, and chemotherapeutic sensitivities, all of which factor into decisions regarding adjuvant chemotherapy. This review considers the questions: (1) Who should be offered adjuvant chemotherapy? (2) What adjuvant chemotherapy should be given? and (3) When should chemotherapy be given?
Who should be considered for adjuvant chemotherapy?
Prognostic Markers
Decisions regarding adjuvant chemotherapy are based on prognosis, that is, risk of breast cancer recurrence, and on the likelihood of benefiting from treatment. A risk of distant recurrence of 10% or more is often used as the threshold at which systemic adjuvant chemotherapy is recommended.
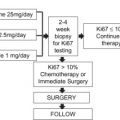
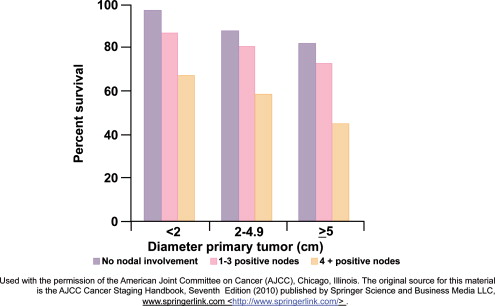
Tumor size and nodal status are important independent prognostic factors for survival for early stage breast cancer ( Fig. 2 ). Other important prognostic factors include histologic grade (based on morphologic features of the tumor), ER, PR (progesterone receptor), HER2 (human epidermal growth factor receptor 2), and the presence of lymphovascular invasion. Several consensus and evidence-based guidelines produced by the National Comprehensive Cancer Network (NCCN), the NIH Consensus Development criteria, and the St Gallen expert opinion criteria provide recommendations on the use of adjuvant chemotherapy in early breast cancer based on clinical data and tumor characteristics.
Adjuvant! Online ( www.adjuvantonline.com ) is a widely used freely available web-based tool. It considers multiple clinical and pathologic factors and produces estimates for recurrence and mortality. In addition, this program incorporates the effect of comorbid conditions in the determination of prognosis and benefit from various therapeutic interventions. Adjuvant! Online has been independently validated by Canadian investigators, and the concordance with actual recurrence and mortality rates was within 1% of predictions based on this model.
Genomic Tests
Several prognostic tests have been developed recently using gene expression profiling. The most widely used commercially available test in the United States is Oncotype Dx (Genomic Health Inc, Redwood City, CA, USA). This reverse transcriptase-polymerase chain reaction (RT-PCR) assay is intended for use in hormone receptor (HR)-positive node-negative breast cancer patients who will receive 5 years of tamoxifen. It measures the gene expression of 16 cancer-related genes (including ER, PR, HER2, and Ki67) in paraffin-embedded tumor tissue and, using a regression model, calculates a recurrence score (RS) that is an estimate of the risk of developing a distant metastases at 10 years. Two suggested cut-off points categorize patients into low (RS <18), intermediate (RS ≥18 <31) and high (RS ≥31) risk groups corresponding to 6.8%, 14.3%, and 30.5% risk of distant recurrence at 10 years after 5 years of tamoxifen therapy, respectively. These risk estimates represent the range of distant recurrence rates for HR-positive, node-negative breast cancers treated with 5 years of tamoxifen.
Recent studies have examined the assay in patients treated with aromatase inhibitors and patients with HR-positive node-positive breast cancer. Other studies have assessed the RS as a tool to predict the risk of locoregional recurrence, the response to neoadjuvant therapy, and finally to predict chemotherapy benefit (specifically for CMF [cyclophosphamide, methotrexate, fluorouracil] and CAF [cyclophosphamide, doxorubicin, fluorouracil]). The ongoing TAILORx (Trial Assigning Individualized Options for Treatment) trial is randomizing patients with HR-positive, node-negative breast cancer and an intermediate RS to chemotherapy and endocrine therapy versus endocrine therapy only. A similar trial for patients with HR-positive, node-positive breast cancer was recently proposed.
Traditional Markers Presented as a Combined Score
Preliminary but very interesting data presented at San Antonio Breast Cancer Symposium 2009 showed that a composite prognostic profile using traditional immunohistochemical markers but measured centrally could provide quantitatively equivalent information as Oncotype DX to clinical information. These data require validation using independent cohorts; nevertheless, they are interesting and highlight the importance of analytical validity and clinical utility for traditional and newer generation prognostic and predictive markers.
The Food and Drug Administration–approved 70-gene signature (Mammaprint, Agendia) was developed at The Netherlands Cancer Institute from a retrospective series of 78 patients younger than 55 years who received no adjuvant systemic therapy and had, node-negative breast cancer of less than 5 cm. This assay stratifies patients into good and poor risk groups according to the risk of developing a distant metastasis. In a study comparing it to Adjuvant! Online, almost 30% of patients had discordant results and for these cases Mammaprint was more accurate at predicting outcome. The prospective multicenter randomized MINDACT (Microarray In Node-negative Disease May Avoid Chemotherapy) trial will test the clinical utility of Mammaprint in selecting patients with node-negative, HR-positive or -negative breast cancer for adjuvant chemotherapy.
There are multiple tools for estimating risk of treatment failure, with and without the use of adjuvant systemic therapies, and some may also predict the magnitude of benefit from endocrine or chemotherapy. All require determination of their clinical utility in prospective trials; while all of them seem to predict outcome, it is uncertain whether there is a “best” predictor.
What adjuvant chemotherapy?
Adjuvant chemotherapy regimens contain non–cross-resistant agents with differing targets and mechanisms of action. There are many regimens considered “standard,” and most were evaluated in clinical trials before the last decade when breast cancer when was considered a single disease entity ( Table 1 ). Selecting the optimum chemotherapy regimen takes into consideration the tumor biology (ER, PR, and HER2 status) and patient factors such as the presence of a comorbidities, for example, congestive heart failure, and peripheral neuropathy. The side effects and toxicities of modern adjuvant chemotherapy are largely transient and reversible; chronic, irreversible side effects (cardiomyopathy, acute myelogenous leukemia, myelodysplastic syndrome) are rare. The following section considers (1) the role of anthracyclines and taxanes in the adjuvant treatment of early breast cancer and (2) how increasingly tumor biology informs chemotherapy choices.
| Regimen | Selected Standard Chemotherapy Regimens |
|---|---|
| Dose-dense AC-T | Doxorubicin 60 mg/m 2 , cyclophosphamide 600 mg/m 2 IV day 1 every 14 days for 4 cycles Followed by Paclitaxel 175 mg/m 2 IV days 1 every 14 days for 4 cycles Granulocyte colony stimulating factor (GCSF) days 3–10 or peg-filgrastim day 2 cycles 1–8 |
| TAC | Docetaxel 75 mg/m 2 IV, doxorubicin 50 mg/m 2 , cyclophosphamide 500 mg/m 2 IV day 1 every 21 days for 6 cycles. GCSF days 3–10 or peg-filgrastim day 2 cycles 1–8 |
| T+(FAC) | Paclitaxel 80 mg/m 2 IV weekly for 12 weeks Followed by Fluorouracil 500 mg/m 2 , doxorubicin 50 mg/m 2 , cyclophosphamide 500 mg/m 2 IV day 1 every 21 days for 4 cycles |
| FEC + Docetaxel | Fluorouracil 500 mg/m 2 , epirubicin 100 mg/m 2 , cyclophosphamide 500 mg/m 2 IV day 1 every 21 days for 3 cycles Followed by Docetaxel 100 mg/m 2 day 1 every 21 days for 3 cycles |
| CEF (Canadian) | Cyclophosphamide 75 mg/m 2 PO days 1–14, epirubicin 60 mg/m 2 IV days 1 and 8, fluorouracil 500 mg/m 2 IV days 1 and 8 every 28 days for 6 cycles With cotrimoxazole support |
| CAF | Cyclophosphamide 100 mg/m 2 day 1, doxorubicin 30 mg/m 2 IV day 1 & 8, fluorouracil 500 mg/m 2 IV day 1 & 8 every 28 days for 6 cycles |
| AC-T | Doxorubicin 60 mg/m 2 IV day 1, cyclophosphamide 600 mg/m 2 IV day 1 Followed by Paclitaxel 80 mg/m 2 IV weekly for 12 weeks |
| TC | Docetaxel 75 mg/m 2 IV day 1, cyclophosphamide 600 mg/m 2 IV day 1 every 21 days for 4 cycles |
| AC | Doxorubicin 60 mg/m 2 , cyclophosphamide 600 mg/m 2 IV day 1 every 21 days for 4 cycles |
| Oral (classic) CMF | Cyclophosphamide 100 mg/m 2 PO days 1–14, methotrexate 40 mg/m 2 days 1–8, fluorouracil 600 mg/m 2 days 1–8 days every 28 days for 6 cycles |
| IV CMF | Cyclophosphamide 100 mg/m 2 IV day 1, methotrexate 40 mg/m 2 IV day 1 and fluorouracil 600 mg/m 2 day 1 IV every 21 days for 9–12 cycles |
Anthracyclines in the Adjuvant Treatment of Early Breast Cancer
Classic CMF (cyclophosphamide, methotrexate, fluorouracil) was one of the first adjuvant polychemotherapy regimens to significantly improve DFS and OS compared with observation in patients with node-positive breast cancer, and remains a widely used regimen. The anthracycline antibiotics (doxorubicin and epirubicin) have been extensively studied in the adjuvant setting beginning in the 1980s mostly in comparison with CMF. The EBCTCG meta-analyses of randomized trials showed that anthracycline-containing regimens were superior to first-generation nonanthracycline-containing regimens such as CMF. Anthracycline-based regimens given for approximately 6 months were shown to reduce the annual breast cancer death rates by approximately 38% for women younger than 50 years at diagnosis and by approximately 20% for women aged between 50 and 69 years at diagnosis.
The effect of anthracycline dose escalation was studied in the Cancer and Leukemia Group B (CALGB) 9344 trial. At doxorubicin doses of 60 mg/m 2 , 75 mg/m 2 and 90 mg/m 2 there was increased toxicity but no improvement in outcome with higher doses. The optimum doses of doxorubicin and epirubicin are considered to be 60 mg/m 2 and 100 mg/m 2 , respectively. Anthracycline-induced cardiac toxicity has been well described. The incidence of congestive heart failure with cumulative doxorubicin doses of 240 to 360 mg/m 2 is between 1.6% and 2.1%. Data from adjuvant breast cancer trials indicate that the risk of congestive cardiac failure is low for women treated with an anthracycline.
Anthracyclines in HER2-Positive Breast Cancer
Over the past 15 years many studies have indicated that the incremental benefit from anthracycline-based therapy is largely confined to HER2-positive breast cancer. A recent meta-analysis reported significant benefit for anthracycline-based regimens in HER2-positive breast cancer in terms of DFS compared to HER2-negative disease. Coamplification of topoisomerase-II-α (TOP2A) and HER2 has been proposed as the mechanism underlying anthracycline sensitivity in HER2-positive beast cancer. TOP2A is one of several targets for anthracyclines and lies in close proximity to HER2 on chromosome 17. Amplification or deletion of TOP2A appears to occur predominately in HER2-positive breast cancer.
Preliminary data from the Breast Cancer International Research Group (BCIRG) 006 trial, which randomized HER2-positive patients to a trastuzumab-containing regimen that contained an anthracycline or did not, reported similar efficacy for both, but fewer cardiac events and secondary leukemias in the nonanthracycline-containing arm. These data have prompted considerable debate regarding the role of anthracyclines in the management of early breast cancer. However, due to the presence of conflicting data and the known existence of unpublished negative studies, HER2 and TOP2A are not considered ready for use in selecting patients for anthracycline therapy. Although there is interest in developing validated and reproducible assays that would help select the most effective drugs or combinations for individual patients, at this time anthracycline-based regimens continue to be the standard of care.
Taxanes in the Adjuvant Treatment of Early Breast Cancer
Activity in the metastatic setting led to clinical trials designed to determine whether the addition of a taxane (paclitaxel or docetaxel) to anthracycline-containing regimens could improve outcome in the adjuvant treatment of breast cancer. These studies have examined sequential and concurrent taxane administration and taxanes as a substitute for an anthracycline. Several meta-analyses have shown small but statistically significant improvements in DFS and OS (approximately 5% and 3% absolute benefit, respectively) favoring the inclusion of a taxane compared with standard anthracycline-containing regimens. Improvements in outcome appear independent of the type of taxane, schedule of administration, hormone receptor, and nodal status.
Randomized Controlled Trials of Taxanes in Early Breast Cancer
The CALGB 9344 trial studied doxorubicin and cyclophosphamide (AC) for 4 cycles followed by paclitaxel (175 mg/m 2 intravenously [IV] every 3 weeks) in node-positive breast cancer compared with AC alone. Improvement in both DFS and OS was observed. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-28 studied sequential paclitaxel (225 mg/m 2 IV every 3 weeks) after 4 cycles of AC in a similar population and observed improved DFS but not OS. Neoadjuvant docetaxel given sequentially with AC was studied in the NSABP B-27 trial and no difference in DFS or OS was observed. However, this trial was underpowered to detect the observed differences with statistical significance.
Several randomized trials examined the efficacy of replacing part of a regimen with a taxane instead of continuing the anthracycline portion. Protocol Adjuvant dans le Cancer du Sein (PACS) 01 trial randomized 1999 node-positive women to 6 cycles of FEC (fluorouracil 500 mg/m 2 , epirubicin 100 mg/m 2 , and cyclophosphamide 500 mg/m 2 ) or 3 cycles of FEC followed by 3 cycles of docetaxel (100 mg/m 2 IV 3 weekly). The addition of docetaxel was associated with an improvement in DFS (hazard ratio, 0.82; 95% CI 0.69–0.99) and OS (hazard ratio, 0.73; 95% CI 0.56–0.94). The GEICAM 9906 study compared 6 cycles of FEC to 4 cycles of FEC followed by paclitaxel for 8 weeks at 100 mg/m 2 . An improvement in DFS (hazard ratio of relapse, 0.77; 95% CI 0.62–0.95; P = .022) and a trend toward improvement in the risk of death (adjusted hazard ratio, 0.78; 95% CI 0.57–1.06; P = .110) in FEC-P compared to FEC was reported.
By contrast, the recently reported UK TACT (Taxotere as Adjuvant Chemotherapy) trial did not report improved outcome with the addition of a taxane. The study randomized more than 4000 pre- and postmenopausal, node-positive or high-risk node-negative patients with operable early breast cancer to FEC (fluorouracil 600 mg/m 2 , epirubicin 60 mg/m 2 , cyclophosphamide 600 mg/m 2 every 3 weeks) for 4 cycles followed by docetaxel (100 mg/m 2 every 3 weeks) for 4 cycles compared with FEC for 8 cycles or epirubicin (100 mg/m 2 every 3 weeks) for 4 cycles followed by CMF (cyclophosphamide 600 mg/m 2 , methotrexate 40 mg/m 2 , and fluorouracil 600 mg/m 2 every 4 weeks) for 4 cycles. There was no benefit observed with the addition of a taxane in terms of DFS (hazard ratio, 0.95; 95% CI 0.85–1.08; P = .44). Suboptimal anthracycline dosing before the delivery of docetaxel may have factored in the lack of reported benefit.
The BCIRG 001 trial compared 6 cycles of FAC to 6 cycles of TAC (docetaxel 75 mg/m 2 IV, doxorubicin 50 mg/m 2 IV, cyclophosphamide 500 mg/m 2 every 3 weeks). Improvement in DFS and OS supporting the replacement of a docetaxel instead of fluorouracil was observed. In the Eastern Cooperative Oncology Group (ECOG) 2197 trial patients were randomized to 4 cycles of AC or 4 cycles AT and no difference in DFS or OS was reported. More recently, studies have examined substituting a taxanes for an anthracycline. The US Oncology clinical trials group compared 4 cycles of AC to 4 cycles of TC and observed a benefit in DFS and OS for TC over AC. An ongoing US Oncology/NSABP clinical trial is currently comparing 6 cycles of TC to 6 cycles of TAC and to 6 cycles of TC plus bevacizumab for 1 year. The NSABP B-30 trial compared (1) 4 cycles of AC followed by 4 cycles of docetaxel (100 mg/m 2 ) every 3 weeks, versus (2) 4 cycles of AT every 3 weeks versus (3) 4 cycles of TAC every 3 weeks. Results from this trial were presented at the 2008 San Antonio Breast Cancer Symposium and demonstrated the superiority of the sequential anthracycline/taxane regimen over the 2 combination regimens. The shorter duration of the combination regimens in the B-30 trial (each 4 cycles total) may have accounted for their inferiority as a similar BCIRG trial that compared 4 cycles of AC followed by 4 cycles of docetaxel (100 mg/m 2 ) every 3 weeks, versus 6 cycles of TAC showed no difference between the two treatment groups.
Preliminary data from the EBCTCG overview analysis, which included more than 20,000 women randomized to a taxane compared with nontaxane-containing regimen, found an improvement in recurrence-free survival (hazard ratio, 0.83, P <.00001) with the addition of a taxane to adjuvant chemotherapy. As with anthracyclines, there is considerable interest in identifying a subgroup particularly sensitive to these agents. Several retrospective studies have observed greater benefit from taxane administration in patients with HER2-positive breast cancer; however, others have not. At this time HER2 status is not used to decide whether a taxane is included or omitted from the adjuvant chemotherapy regimen. Based on the known mechanism of action of taxanes, several other biomarkers have been proposed as potential predictors of response or resistance, for example, overexpression of p -glycoprotein, a drug efflux pump, and alterations in the structure of β-tubulin and microtubule associated proteins (MAP), (eg, MAP-Tau). At present no established single gene markers exist that could identify a patient population that is particularly sensitive to these agents.
Taxane Scheduling
The ECOG 1199 study provided evidence that the scheduling of taxane treatment is important. In this study almost 5000 patients with node-positive breast cancer were randomized in a 2-by-2 factorial design to 4 different taxane regimens—(1) 4 cycles of paclitaxel 175 mg/m 2 every 3 weeks, (2) 12 cycles of paclitaxel 80 mg/m 2 given weekly, (3) 4 cycles of docetaxel 100 mg/m 2 every 3 weeks, or (4) 12 cycles of docetaxel 35 mg/m 2 given weekly—after completion of 4 cycles of AC. The DFS rates were 76.9% for 3-weekly paclitaxel; 81.5% for weekly paclitaxel; 81.2% for 3-weekly docetaxel; and 77.6% for weekly docetaxel. Improvements in OS were observed for weekly paclitaxel and 3-weekly docetaxel in comparison with paclitaxel given every 3 weeks. Toxicities in the weekly docetaxel arm, in particular hematological, led to administration of fewer cycles of treatment.
Dose Density
Gompertzian growth kinetics display increased doubling time and decreased growth fraction as a function of time, and provide the rationale for dose-dense chemotherapy. The Norton-Simon hypothesis argues that smaller rapidly dividing tumors are more sensitive to cytotoxic chemotherapy, and that by shortening the interval between each cycle of chemotherapy there is less time for tumor regrowth and therefore greater fraction of cell kill per cycle. This strategy has been studied in several randomized controlled trials. The CALGB 9741 randomized, 2005 node-positive patients to standard doses of sequential A, T, C, or concurrent AC followed by T in a 2-by-2 factorial design. Each regimen was administered every 3 weeks or at dose-dense intervals every 2 weeks with granulocyte colony-stimulating factor support. The same number of drug cycles and the same cumulative dose of each drug were administered to all patients. An improvement in DFS and OS was observed with the dose-dense strategy. In the NCIC MA.21 study at a median follow-up of 30 months, AC-T given every 3 weeks was inferior to both dose-dense EC-T and CEF for 6 cycles. Dose-dense AC-T has been compared with TAC in the NSABP B-38 trial. These results are not available as yet.
What adjuvant chemotherapy?
Adjuvant chemotherapy regimens contain non–cross-resistant agents with differing targets and mechanisms of action. There are many regimens considered “standard,” and most were evaluated in clinical trials before the last decade when breast cancer when was considered a single disease entity ( Table 1 ). Selecting the optimum chemotherapy regimen takes into consideration the tumor biology (ER, PR, and HER2 status) and patient factors such as the presence of a comorbidities, for example, congestive heart failure, and peripheral neuropathy. The side effects and toxicities of modern adjuvant chemotherapy are largely transient and reversible; chronic, irreversible side effects (cardiomyopathy, acute myelogenous leukemia, myelodysplastic syndrome) are rare. The following section considers (1) the role of anthracyclines and taxanes in the adjuvant treatment of early breast cancer and (2) how increasingly tumor biology informs chemotherapy choices.
| Regimen | Selected Standard Chemotherapy Regimens |
|---|---|
| Dose-dense AC-T | Doxorubicin 60 mg/m 2 , cyclophosphamide 600 mg/m 2 IV day 1 every 14 days for 4 cycles Followed by Paclitaxel 175 mg/m 2 IV days 1 every 14 days for 4 cycles Granulocyte colony stimulating factor (GCSF) days 3–10 or peg-filgrastim day 2 cycles 1–8 |
| TAC | Docetaxel 75 mg/m 2 IV, doxorubicin 50 mg/m 2 , cyclophosphamide 500 mg/m 2 IV day 1 every 21 days for 6 cycles. GCSF days 3–10 or peg-filgrastim day 2 cycles 1–8 |
| T+(FAC) | Paclitaxel 80 mg/m 2 IV weekly for 12 weeks Followed by Fluorouracil 500 mg/m 2 , doxorubicin 50 mg/m 2 , cyclophosphamide 500 mg/m 2 IV day 1 every 21 days for 4 cycles |
| FEC + Docetaxel | Fluorouracil 500 mg/m 2 , epirubicin 100 mg/m 2 , cyclophosphamide 500 mg/m 2 IV day 1 every 21 days for 3 cycles Followed by Docetaxel 100 mg/m 2 day 1 every 21 days for 3 cycles |
| CEF (Canadian) | Cyclophosphamide 75 mg/m 2 PO days 1–14, epirubicin 60 mg/m 2 IV days 1 and 8, fluorouracil 500 mg/m 2 IV days 1 and 8 every 28 days for 6 cycles With cotrimoxazole support |
| CAF | Cyclophosphamide 100 mg/m 2 day 1, doxorubicin 30 mg/m 2 IV day 1 & 8, fluorouracil 500 mg/m 2 IV day 1 & 8 every 28 days for 6 cycles |
| AC-T | Doxorubicin 60 mg/m 2 IV day 1, cyclophosphamide 600 mg/m 2 IV day 1 Followed by Paclitaxel 80 mg/m 2 IV weekly for 12 weeks |
| TC | Docetaxel 75 mg/m 2 IV day 1, cyclophosphamide 600 mg/m 2 IV day 1 every 21 days for 4 cycles |
| AC | Doxorubicin 60 mg/m 2 , cyclophosphamide 600 mg/m 2 IV day 1 every 21 days for 4 cycles |
| Oral (classic) CMF | Cyclophosphamide 100 mg/m 2 PO days 1–14, methotrexate 40 mg/m 2 days 1–8, fluorouracil 600 mg/m 2 days 1–8 days every 28 days for 6 cycles |
| IV CMF | Cyclophosphamide 100 mg/m 2 IV day 1, methotrexate 40 mg/m 2 IV day 1 and fluorouracil 600 mg/m 2 day 1 IV every 21 days for 9–12 cycles |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree