Summary of Key Points
- •
Surgery remains the most effective curative approach for early-stage (IA–IIB) NSCLC. Selected cases with clinical stage IIIA disease equally benefit from radical resection.
- •
Stereotactic radiotherapy may be an alternative for selected medically inoperable patients.
- •
Long-term survival after surgical resection is stage-related, with the likelihood of recurrence increasing with advancing cancer stage (of course this is what staging is all about).
- •
Two comprehensive systematic reviews and meta-analyses, recently updated, showed a significant benefit of adding chemotherapy after surgery, with an absolute increase in survival of 4% at 5 years. The other meta-analysis in which surgery plus radiotherapy and chemotherapy was compared with surgery and radiotherapy showed indeed a significant benefit, representing an absolute improvement in survival of 4% at 5 years.
- •
The role of adjuvant radiotherapy for patients with N2 disease remains unclear, and an ongoing study is addressing the issue.
- •
The role of molecular targeted therapies currently remains generally unproven; studies investigating bevacizumab and epidermal growth factor receptor inhibitors in an imprecisely defined patient population did not show meaningful benefit.
- •
There is no clear role for molecular prognostic and predictive biomarkers or molecular signatures to assist in treatment selection.
Lung cancer is the most common fatal malignant disease among men and women worldwide. Approximately 90% of lung cancers are tobacco-related. Primary prevention of lung cancer through smoking cessation is the first goal in reducing the incidence of lung cancer. However, tobacco consumption is increasing worldwide and the risk of lung cancer is higher for former smokers than for never-smokers; in the United States, more than 50% of lung cancers occur in former smokers. Consequently, lung cancer will continue to be a relevant health problem for the foreseeable future.
More than 80% of all newly diagnosed cases of lung cancer are nonsmall cell lung cancer (NSCLC). Surgery is the main curative therapeutic approach for early-stage NSCLC (stages IA to IIB), but early-stage NSCLC is only a minority (20% to 25%) of all cases. Some groups of patients with stage III disease also benefit from pulmonary resection, usually in combination with other treatment modalities.
Long-term survival after surgical resection is stage-related, with the likelihood of recurrence increasing with advancing cancer stage. One-third of patients with stage IA will relapse and die of the disease within 5 years. Relapse occurs after resection in more than 50% of patients with stage II NSCLC. The majority of these relapses are distant metastases, with a 10% risk of a local recurrence after complete resection. The brain is the most common site of metastatic recurrence, followed closely by bone, ipsilateral and contralateral lung, the liver, and adrenal glands. Histology influences the pattern of recurrence; local recurrence is more common in patients with squamous cell carcinoma and distant metastases are more likely in patients with adenocarcinoma ( Table 51.1 ). More than 80% of recurrences occur within 2 years after radical surgery. A 2010 investigation of the timing of local and distant failures showed that among 975 patients with stage I or II disease, recurrent disease developed in 250 patients: 43 at local sites, 110 at distant sites, and 97 at both local and distant sites. The median times to local and distant failure were 13.9 months and 12.5 months, respectively (range, 1 to 79 months for both types of failure). In most patients who had both local and distant recurrence, the failure occurred at both sites simultaneously. This finding is important because only time to first failure has been reported in many trials, and additional sites of failure have not been subsequently analyzed. These results support the integration of local treatment modalities with systemic therapies.
| Pattern of Relapse (%) | ||||
|---|---|---|---|---|
| Author (Year) | Stage | No. of Patients | Local–Regional Only | Distant Only |
| Martini et al. (1980) | T1–2 N1 (S) T1–2 N1 (A) T2–3 N2 (S) T2–3 N2 (A) | 93 114 46 103 | 16 8 13 17 | 31 54 52 61 |
| Feld et al. (1984) | T1 N0 T2 N0 T1 N1 | 162 196 32 | 9 11 9 | 17 30 22 |
| Pairolero et al. (1984) | T1 N0 T2 N0 T1 N1 | 170 158 18 | 6 6 28 | 15 23 39 |
| Thomas et al. (1990) | T1 N0 (S) T1 N0 (NS) | 226 346 | 5 9 | 7 17 |
Micrometastatic dissemination of cancer cells at levels that are undetectable with currently available imaging techniques seems to affect the prognosis of patients with clinical early-stage NSCLC. It has been shown consistently that positron emission tomography (PET), which is now routinely included in the staging workup for NSCLC, detects metastatic disease in 11% to 14% of cases otherwise cleared for resection using conventional screening methods and also better detects unsuspected disease in the mediastinal and hilar nodes. Despite improved detection with PET, micrometastases are missed. In small retrospective studies, researchers have attempted to detect micrometastatic lymph node disease with immunohistochemistry (IHC) and real-time polymerase chain reaction to identify cytokeratins and carcinoembryonic antigens. Patients with positive findings in otherwise morphologically normal lymph nodes were almost invariably more likely to have adverse outcomes than patients without occult micrometastatic disease. Quantification of free circulating DNA has been proposed as a potential additional diagnostic tool for use in patients with resected or persisting neoplastic disease.
As screening techniques are incorporated into preventive and primary care models, it is hoped that the pattern of lung cancer diagnoses can be shifted from stage IV to earlier stages, leading to further interest in the use of systemic adjuvant therapies. This stage migration will be important for decreasing the mortality of lung cancer; however, as we increase the number of patients detected with stage I disease, we must also be able to have some noninvasive molecular or imaging modalities that will help to identify patients with stage I disease who may benefit from additional local and systemic treatments after a complete resection to improve long-term survival.
Rationale for Adjuvant Therapy
The use of adjuvant (postoperative) therapy for the treatment of various solid tumors is well established and is based on theoretical models and clinical observations. After complete resection, a patient’s tumor load should be nonexistent or minimal. Any residual neoplastic cells present in micrometastatic deposits should contain few clones resistant to chemotherapy or radiotherapy. Experimental and clinical data support the Gompertzian model of tumor growth and regression in most human solid cancers: when a tumor is present microscopically but is clinically undetectable, its growth rate should be at its highest. Therefore, although the numerical reduction of malignant cells induced by cytotoxic chemotherapy is small, the fractional cell kill from an effective dose of chemotherapy should be high.
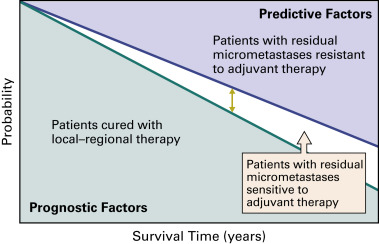
The decision to use adjuvant therapy involves balancing the need to treat a large number of patients who may be cured by surgery alone against the need for additional systemic therapy to eradicate remaining cancer cells in only a subset of these patients. Thus, if survival is increased for 10% of patients, the other 90% are exposed unnecessarily, either because they did not need the adjuvant treatment or because adjuvant therapy was ineffective in eradicating residual disease. Because no tools exist that determine prospectively who will benefit from adjuvant therapy, it is very important to select a tolerable regimen and limit the length of treatment ( Fig. 51.1 ). In addition, careful pathologic staging enables better prediction of prognosis, facilitates patient selection, and permits comparison of treatment outcomes among trials.
The most appropriate treatment or regimen for the adjuvant setting has not been established. At a minimum, the chosen agent or agents should have proven activity in advanced disease and should be generally well tolerated. Thus, in the case of cytotoxic chemotherapy, a platinum-based doublet regimen should be selected, initiated sufficiently early after radical surgery, and administered for at least three or four cycles.
Role of Adjuvant Radiotherapy
Before effective chemotherapy regimens were established, postoperative thoracic radiotherapy was the preferred adjuvant treatment. Although radiotherapy may improve local–regional control, it is unlikely to reduce systemic recurrence. Use of radiotherapy has been evaluated in many retrospective and prospective studies. Data from nine of these studies (2128 patients) were included in the Postoperative Radiation Therapy (PORT) meta-analysis. The authors of this meta-analysis concluded that postoperative radiotherapy had a significant detrimental effect on survival, especially for patients with stage I and II disease. These results were confirmed by a Cochrane systematic review and meta-analysis in 2000 and substantially updated in 2005, which demonstrated that postoperative radiotherapy may have a significant adverse effect on survival (hazard ratio [HR], 1.18). The 18% relative increase in the risk of death is equivalent to an absolute detriment of 6% at 2 years (95% CI, 2% to 9%), reducing overall survival (OS) from 58% to 52%. Exploratory subgroup analyses indicate that this detrimental effect was most pronounced for patients with stage I or II disease. For patients with stage III N2 disease, there was no clear evidence of an adverse effect or potential benefit. This outcome is plausible because increased frequency of local–regional failure is associated with the bulky disease often seen in stage III NSCLC.
In most of the studies included in the PORT meta-analysis, patients were treated with older radiotherapy technology (e.g., cobalt 60) and outdated dosimetry, which are less effective than current treatment approaches, and the higher mortality rate in the radiotherapy groups can be attributed in part to an excess of deaths related to intercurrent disease. In a retrospective review, it was reported that use of new technologies and improved dosimetry for postoperative radiotherapy does not excessively increase the risk of death related to intercurrent disease. Another shortcoming of the PORT meta-analysis is that it failed to include sufficient data on mediastinal lymph node dissection, and, additionally, the surgical procedure varied substantially across studies and centers.
Using data from the United States Surveillance, Epidemiology and End Results (SEER) database, researchers evaluated the relation between survival and postoperative radiotherapy. Factors with a negative effect on OS were older age, T3 or T4 tumor stage, N2 node stage, male gender, fewer lymph nodes sampled, and a greater number of involved lymph nodes. In this study, the use of postoperative radiotherapy was associated with increased survival for patients with N2 disease, but not for patients with N1 or N0 disease.
The role of radiotherapy for patients with N2 disease remains unclear, as no definitive conclusions can be drawn from the available literature. The Lung Adjuvant Radiation Therapy trial in Europe, still ongoing, includes patients with N2 NSCLC who have had surgery, with or without adjuvant chemotherapy. Patients are randomly assigned to postoperative radiotherapy (54 Gy) or no radiotherapy, and it is hoped that results from this trial will define the role of adjuvant radiotherapy for patients with N2 NSCLC.
Early Studies of Adjuvant Chemotherapy
In the 1960s and 1970s, alkylating agents and nonspecific immunotherapies (e.g., levamisole and bacillus Calmette–Guerin vaccine) universally failed, and detrimental effects of these agents were occasionally reported. These drugs are now known to have very limited or no activity in advanced NSCLC. Subsequently, the use of cisplatin-based chemotherapy was extensively tested in all stages of resectable NSCLC. In all but one of these early studies, adjuvant therapy failed to show clinical benefit. Common flaws in the design of these trials were overestimation of the potential benefit of adjuvant chemotherapy, imbalance in relevant patient and treatment characteristics (for instance, the rate of incomplete mediastinal lymph node dissection), and unrealistic patient accrual goals. In addition, in most of the trials, chemotherapy dose delivery (total dose and dose intensity) was often inadequate, with only 50% of patients, on average, receiving the intended course of treatment. Given the toxicity of these regimens in the absence of good antiemetic supportive care and the lack of proven survival benefit from adjuvant therapy, physicians were reluctant to offer participation in adjuvant trials to their patients.
Nevertheless, the authors of a large meta-analysis, which included these trials, reported a 13% reduction in the risk of death with adjuvant cisplatin-based chemotherapy, a result of borderline significance ( p = 0.08). There was a 6% reduction in the risk of death among patients who received postoperative radiotherapy and cisplatin-based chemotherapy compared with patients who received postoperative radiotherapy only ( p = 0.46). In contrast, adjuvant chemotherapy with alkylating agents was shown to be significantly detrimental (HR, 1.15; p = 0.005).
These findings failed to have an effect on clinical practice because they were of only borderline significance and were based on several flawed studies. In addition, the heterogeneity of surgical procedures and the difference in the staging modalities limited the applicability of the results. Nevertheless, the findings strongly supported a potential role for adjuvant chemotherapy and the need for a large, well-designed confirmatory trial.
Large-Scale Studies on Platinum-Based Adjuvant Chemotherapy
Given the evidence of a marginal benefit of adjuvant systemic treatment, several randomized studies were conducted to evaluate the role of modern, platinum-based regimens in all stages of resectable NSCLC ( Table 51.2 ). Some of the trials were initiated prior to the publication of the PORT meta-analysis and thus included postoperative radiotherapy.
| ALPI/EORTC | IALT | BLT | ANITA | NCIC-JBR.10 | CALGB 9633 | |
|---|---|---|---|---|---|---|
| Regimen | Cisplatin, vindesine, and mitomycin (3 cycles) | Cisplatin and vindesine, vinblastine, vinorelbine, or etoposide (3–4 cycles) | Cisplatin and vindesine; cisplatin and vinorelbine; cisplatin, vinblastine, and mitomycin; or cisplatin, mitomycin, and ifosfamide (3 cycles) | Cisplatin and vinorelbine (4 cycles) | Cisplatin and vinorelbine (4 cycles) | Carboplatin and paclitaxel (4 cycles) |
| Sequential radiotherapy allowed | Yes | Yes | Yes | Yes | No | No |
| No. of patients enrolled/planned | 1209/1300 | 1867/3300 | 381/500 | 840/800 | 482/450 | 344/384 b |
| Median age (y) | 61 | 59 | 61 | 59 | 61 | 61 |
| Male:female ratio | 86:14 | 81:19 | 65:35 | 85:14 | 66:34 | 65:35 |
| Stage (%) | ||||||
| I II IIIA | 39 31 29 | 37 24 39 | 29 37 27 | 36 a 24 39 | 46 54 0 | 100 a (all IB) |
| Histology (%) Squamous Nonsquamous | 51 45 | 47 46 | 48 52 | 59 40 | 37 65 | 35 65 |
| Rate of pneumonectomy (%) | 26 | 35 | NR | 38 | 25 | 11 |
b The original sample size was 500 patients and was subsequently emended. The study was closed early based on the recommendation of the Data Safety Monitoring Board.
The first of these studies to be reported was an Eastern Cooperative Oncology Group (ECOG) trial, which compared the efficacy of four cycles of adjuvant cisplatin and etoposide plus concomitant thoracic radiotherapy (total dose of 50 Gy) with postoperative radiotherapy alone for 488 patients with stage II and IIIA disease. There was no significant difference between the two treatment arms in terms of median time to progression. The median survival was 38 months for the concurrent chemoradiation therapy arm and 39 months for radiotherapy alone arm (HR, 0.93; 95% CI, 0.74–1.18). The lack of efficacy may have been due to the toxicity of radiation with concomitant administration of cytotoxic agents; this effect was more striking in patients with stage II disease. In a biologic correlative study of 197 tumors from this trial, neither p53 protein expression nor Kirsten rat sarcoma viral oncogene homolog ( KRAS ) mutation showed any relation to outcome.
In a joint effort, the Adjuvant Lung Project Italy (ALPI) and the European Organisation for Research and Treatment of Cancer (EORTC) enrolled 1209 patients with completely resected stage I, II, or IIIA NSCLC between 1994 and 1999. Patients were randomly assigned to either three cycles of chemotherapy with mitomycin, vindesine, and cisplatin (MVP) or to observation. Sixty-nine percent of patients completed the three cycles of MVP, with half of those patients needing dose reductions. Radiotherapy was administered sequentially according to the policy at each center, and 43% of patients received postoperative radiotherapy. There was no significant difference in OS between the two groups (HR for death, 0.96). The median OS was 55 months in the chemotherapy arm and 48 months in the observation arm (HR, 0.96; 95% CI, 0.81–1.13; p = 0.59). Subset analysis by stage showed that 5-year survival was better for patients with stage II disease than for patients with stage I or III disease ( Table 51.3 ). Even though the HR for patients with stage II NSCLC was not significant, it is notable that, in this subset of patients, there was a 10% survival advantage at 5 years for patients who received chemotherapy. No significant association was found between p53 or Ki67 expression, and disease stage or tumor histology. The relation of KRAS mutation status to survival was analyzed using specimens from adenocarcinomas and large-cell carcinomas; mutations were found in 22% of 117 samples, with no relation to survival.
| Hazard Ratio for 5-Year Survival (95% CI) | ||||
|---|---|---|---|---|
| Study | Overall | Stage I | Stage II | Stage III |
| ALPI/EORTC | 0.96 (0.81–1.13) p = 0.59 | 0.97 (0.71–1.33) | 0.80 (0.60–1.06) | 1.06 (0.82–1.38) |
| IALT | 0.86 (0.76–0.98) p < 0.03 | 0.95 (0.74–1.23) | 0.93 (0.72–1.20) | 0.79 (0.66–0.95) |
| BLT | 1.02 (0.77–1.35) p = 0.90 | NT | NT | NT |
| ANITA | 0.80 (0.66–0.96) p = 0.017 | 1.14 (0.83–1.57) | 0.67 0.47–0.94) | 0.60 (0.44–0.82) |
| NCIC JBR.10 | 0.69 (0.52–0.91) p = 0.04 | 0.94 | 0.59 (0.42–0.85) | NI |
| CALGB 9633 a | — | 0.62 (0.41–0.95) p = 0.028 | NI | NI |
a Early data, collected after a median follow-up of 34 months.
The International Adjuvant Lung Cancer Trial (IALT) was the first large trial to demonstrate a significant benefit of adjuvant chemotherapy. A total of 1867 patients with completely resected NSCLC were randomly assigned to cisplatin plus a second drug (vindesine, vinblastine, vinorelbine, or etoposide) or to observation. Approximately 10% had stage IA disease, 27% had stage IB, 24% had stage II, and 39% had stage III. In the chemotherapy arm, 74% of patients received at least 240 mg/m of cisplatin and 27% of patients received postoperative radiotherapy. Grade 3 or 4 toxicity was reported for 23% of patients (0.8% of toxicity-related deaths). Survival was significantly longer in the chemotherapy arm (HR, 0.86; 95% CI, 0.76–0.98; p < 0.03): 5-year survival in the chemotherapy and observation arms were 44.5% and 40.4%, respectively. The median survival was 50.8 months and 44.4 months, respectively, and the median disease-free survival was 40.2 months and 30.5 months ( Table 51.3 ).
In the Big Lung Trial (BLT), 381 patients with resected stage I–III NSCLC were randomly assigned to three cycles of postoperative chemotherapy (cisplatin and vindesine; cisplatin, mitomycin, and ifosfamide; cisplatin, mitomycin, and vinblastine; or cisplatin and vinorelbine) or to surgery alone. Sixty-four percent of patients received all three courses of chemotherapy, and 40% of them needed dose reductions. Postoperative radiotherapy was used for only 14% of patients. No significant differences in survival were noted between the two groups. However, this trial was underpowered, had a short follow-up (29 months), and a 15% rate of incomplete resection.
In the Adjuvant Navelbine International Trial Association (ANITA) trial, 840 patients with resected stage IB–IIIA NSCLC were randomly assigned to cisplatin (100 mg/m 2 ) every 4 weeks and vinorelbine (30 mg/m 2 ) weekly or to observation; 301 patients (36%) had stage IB disease, 203 (24%) had stage II disease, and 325 (39%) had stage IIIA disease. After a median follow-up of 76 months, the median survival was 65.7 months in the chemotherapy group and 43.7 months in the observation group. Overall, chemotherapy significantly reduced the risk of death (HR, 0.80; 95% CI, 0.66–0.96; p = 0.017) and conferred a survival advantage of 8.6% at 5 years, which was maintained at 7 years (8.4%). The 5-year survival rate was better for patients with stage III disease than for patients with stage I or II disease ( Table 51.3 ). Grade 3 or 4 neutropenia was documented for 85% of patients, febrile neutropenia for 9%, and severe infection for 11%. The most common nonhematologic adverse effects were asthenia (28%), nausea and vomiting (27%), and anorexia (15%). As in many of the other studies of adjuvant chemotherapy already described, postoperative radiotherapy was administered according to the policy of individual centers. Radiotherapy was beneficial for patients with N2 disease and harmful for patients with N1 disease when combined with chemotherapy.
The role of adjuvant chemotherapy in the treatment of stage I and II NSCLC was investigated in the National Cancer Institute of Canada Clinical Trials Group JBR.10 trial, which was powered to detect a 10% improvement in 3-year survival. Four hundred and eighty-two patients with resected stage IB and II NSCLC (excluding T3 N0) were enrolled and randomly assigned to receive four cycles of cisplatin (50 mg/m 2 ) on days 1 and 8 every 4 weeks and vinorelbine (25 mg/m 2 ) weekly for 16 weeks or to observation. Patients did not receive postoperative radiotherapy, and they were stratified by node status (N0 or N1) and KRAS mutation status. OS was significantly longer in the chemotherapy arm (94 vs. 73 months; HR, 0.69; 95% CI, 0.52–0.91; p = 0.04), as was recurrence-free survival (not reached vs. 47 months; HR, 0.60; p < 0.001). The 5-year survival rate was 69% in the chemotherapy arm and 54% in the observation arm ( p = 0.03), with an absolute gain of 15% at 5 years. In the subset analysis by stage, patients with stage II disease had a greater survival benefit at 5 years (difference of 20% between study arms, p = 0.004) than patients with stage I disease (7% difference between study arms; not significant) ( Table 51.3 ). Fifty-eight percent of the 231 patients who received chemotherapy received three cycles of cisplatin and vinorelbine. Nineteen percent of patients were hospitalized for problems related to chemotherapy toxicity. In an updated analysis of this study with a median follow-up of 9.3 years, adjuvant chemotherapy continued to produce a significant survival benefit ( p = 0.04), with an absolute improvement of 11% in 5-year survival (67% in the chemotherapy arm vs. 56% in the observation arm) ( Table 51.4 ). The benefit was particularly pronounced for patients with stage II disease (median survival, 6.8 vs. 3.6 years), and there was no survival benefit for patients with stage IB disease (median survival, 11.0 vs. 9.8 years). However, within the population with stage IB disease, tumor size was predictive of chemotherapy effect, with chemotherapy of benefit for patients with tumors 4 cm or larger (HR, 0.66) compared with patients with smaller tumors (HR, 1.73). The 5-year survival for patients with tumors 4 cm or larger was 79% for patients in the chemotherapy arm compared with 59% for patients in the observation arm. KRAS mutation was not associated with a differential effect of chemotherapy.
| Study | Follow-Up (Y) | Hazard Ratio for Overall Survival (95% CI) |
|---|---|---|
| NCIC JBR.10 | 9.3 | 0.78 (0.61–0.99) p = 0.04 |
| IALT | 7.5 | 0.91 (0.81–1.02) p = 0.1 |
| CALGB 9633 | 6.2 | 0.83 (0.64–1.08) p = 0.125 |
Similarly, long-term data from the IALT trial were reported at a median follow-up of 7.5 years and the OS advantage was no longer significant ( p = 0.1) ( Table 51.4 ). This late loss of survival benefit appears to be due to an excess of noncancer-related deaths in the chemotherapy arm.
Unfortunately, JBR.10 and ANITA, two of the positive studies, used chemotherapy doses and schedules that are not routinely used in current clinical practice. The most common dose of cisplatin currently used is 75 mg/m 2 on day 1; cisplatin on days 1 and 8 (as in the JBR-10 trial) is unusual and weekly vinorelbine for 16 weeks is associated with high toxicity and a challenge to safely administer in the adjuvant setting. These trials provide the necessary clinical evidence for adjuvant chemotherapy and may justify the use of other cisplatin-based doublets with similar activity for stage IV NSCLC at the same doses and schedules.
The Cancer and Leukemia Group B (CALGB) 9633 trial was unique in limiting enrollment to patients with resected stage IB disease and is the only large trial to have used a carboplatin-based regimen. The study was powered to detect a 13% improvement in OS at 5 years. In this study, 344 patients were randomly assigned to receive carboplatin (AUC 6) and paclitaxel (200 mg/m 2 ) every 3 weeks for a total of four cycles, and the trial was closed early (90% of patients recruited) when an interim analysis showed a 12% absolute improvement in OS at 4 years (71% vs. 59%; HR, 0.62; p = 0.028). Chemotherapy delivery was excellent, with nearly 85% of patients receiving four cycles of chemotherapy. Toxicity in this group of patients was minimal, with 36% of patients having grade 3 or grade 4 myelosuppression and no treatment-related deaths.
Following initial closure and early reporting, the final analysis of the results of this trial could not confirm a significant favorable outcome. After an extended follow-up (74 months), there was only a nonsignificant trend toward improvement in survival (59% vs. 57%; p = 0.125) ( Table 51.4 ). It should be noted that the small sample size did not allow for adequate power to detect small differences in survival. The 3-year failure-free survival (66% vs. 57%) and the 3-year OS (79% vs. 70%; p = 0.045) continued to favor the chemotherapy group.
A pooled analysis of data from JBR.10 and CALGB 9633 was done to evaluate survival according to tumor size. The authors found that the effect of chemotherapy seemed to increase with tumor size. Thus, current entry criteria for adjuvant chemotherapy trials in North America specify a tumor size of 4 cm or larger. The impact of adjuvant chemotherapy use and tumor size on outcomes in stage I has been reviewed from 2003 to 2006 in the National Cancer Database in the United States. The results of the study indicate an increased use over time of adjuvant chemotherapy although it continues to frequently not be used. The analysis also supports the guidelines indicating adjuvant chemotherapy for stage I NSCLC tumors larger than 4 cm with a positive survival impact for patients whose tumors ranged from 3.0 to 8.5 cm.
Overall three studies showed a positive impact of adjuvant chemotherapy in resectable NSCLC, with a survival benefit ranging from 4.1% (IALT) to 15% (JBR.10). A few factors may help to explain the difference in survival among the landmark adjuvant studies ( Table 51.3 ). First, the sample size differed substantially among the studies (from less than 500 patients to 3300 according to the planned sample sizes) to assess the same expected therapeutic effect in the same patient population. Effectively, the only two studies designed to detect a reasonable survival advantage were the ALPI and IALT trials, which, not surprisingly, demonstrated a relatively similar survival benefit: 3% and 4.1%, respectively.
Second, most of the landmark adjuvant studies do not include information about the proportion of patients who had systematic lymph node dissection or lymph node sampling. This detail is important, as one randomized clinical study showed that systematic lymph node dissection significantly influenced survival for every stage of resectable NSCLC. Third, patients with lung cancer frequently have comorbidities, including chronic obstructive pulmonary disease and cardiovascular diseases, which can affect survival substantially. Lastly, an unbalance in the proportion of patients who quit smoking after radical surgery may potentially account for survival differences, as was shown in two retrospective studies. A common feature of most of these landmark studies, with the exclusion of the CALGB 9633, is the less than optimal compliance with adjuvant regimens. Because of treatment delays and dose reductions, the delivery of three cycles of adjuvant chemotherapy ranged from 58% to 74% in studies of cisplatin-based chemotherapy. Reasons for low rates of treatment compliance may be related to the time needed to fully recover from the surgical procedure for lung cancer (which is longer than the time to recover from breast cancer surgery, for example). The rate of pneumonectomy in some of these studies far exceeded the rate in consecutive surgical series; pneumonectomy was done in 26% of patients in ALPI, in 35% of patients in IALT, and in 41% of patients in ANITA, and a subset analysis of the tolerability of chemotherapy in these subgroups has not been performed. Of note, in breast cancer, the survival benefit of adjuvant therapy has been more striking in patients who receive more than 85% of the intended total dose of chemotherapy.
The impact of platinum-based adjuvant chemotherapy on survival has also been specifically evaluated in Asian patients with stage I–IIIA NSCLC. Among 2231 patients in the Taiwan cancer registry who had resection in 2004 to 2007, the mortality rate was lower for patients treated with chemotherapy for both stage II and IIIA disease. Multivariate analysis demonstrated that platinum-based adjuvant chemotherapy was an independent prognostic factor for OS for patients with stage II disease ( p = 0.024), including both men and women and patients older than 70 years of age.
Quality of Life and Adjuvant Cisplatin-Based Chemotherapy
The impact of adjuvant chemotherapy on quality-of-life outcomes has also been investigated. This specific outcome was evaluated in the JBR.10 trial through the EORTC quality-of-life questionnaire C30 and a trial-specific checklist at baseline and at weeks 5 and 9 for patients who received chemotherapy and for all patients at regular follow-up visits. The impact of initial surgery on quality of life was similar for the two treatment arms (chemotherapy and observation), whereas the quality of life during chemotherapy was only modestly affected, (in particular, for fatigue, nausea, and vomiting), but without associated changes in global quality of life. These symptoms improved considerably at 3 months of follow-up, with more permanent side effects being limited to sensory neuropathy and chemotherapy-associated hearing loss. Thus, negative effects on quality of life appear to be modest and fairly short-lived. Investigators further followed up on these findings by assessing quality-adjusted time without symptoms or toxicity and reported that adjuvant chemotherapy was preferred for relapse and toxicity and had an overall better quality-adjusted time in the range of 5 to 6 additional months.
Studies of Adjuvant Treatment With Oral UFT
UFT, an oral fluoropyrimidine combining uracil and tegafur, has been extensively studied in Japan as adjuvant treatment as a single-agent or in combination with intravenous cytotoxic agents. In the largest trial of postoperative UFT therapy in resected NSCLC, by Kato et al., 979 patients with completely resected stage I adenocarcinoma were randomly assigned to either oral UFT (250 mg/m 2 ) for 2 years or to observation. OS favored the UFT arm (HR, 0.71; 95% CI, 0.52–0.98; p = 0.04) and the 5-year survival was 88% in the UFT arm compared with 85% in the observation arm. Subset analyses showed that the benefit was greatest for the subgroup of 263 patients who had T2 N0 disease (HR, 0.48; 95% CI, 0.29–0.81; p = 0.005) but not for the 716 patients who had T1 N0 disease (HR, 0.97; 95% CI, 0.64–1.46; p = 0.87). Compliance was limited to 74% at 12 months and 61% at 24 months. One questionable point in this trial is the absence of any advantage in disease-free survival for the patients in the UFT arm, and this finding clearly contrasts with the results of the positive studies of cisplatin-based adjuvant therapy (IALT, JBR.10, and ANITA), in which improvement in OS for patients receiving adjuvant chemotherapy was invariably associated with disease-free survival that was similar or of greater magnitude.
The results of other published studies of adjuvant UFT in smaller patient cohorts were completely or partially inconsistent with the data found in the study by Kato et al., and confirmatory data for white patients are lacking. Additionally, questions regarding a specific genetic sensitivity to UFT in Japanese patients remain unanswered.
Efficacy of Adjuvant Chemotherapy According to Systematic Reviews, Meta-Analyses, and Cancer Registry Data
Several systematic reviews and meta-analyses have confirmed the value of adjuvant cisplatin- or UFT-based chemotherapy for resected NSCLC ( Table 51.5 ). All of these reviews have consistently shown a benefit from adjuvant chemotherapy, with HRs ranging from 0.72 for adjuvant UFT to 0.89 for cisplatin-based chemotherapy.
| Author | Adjuvant Treatment | Number of Studies (Patients) | Hazard Ratio (95% CI) for Overall Survival |
|---|---|---|---|
| Hotta et al. | Cisplatin-based chemotherapy Single-agent UFT | 8 (3786) 5 (1751) | 0.89 (0.81–0.97) 0.79 (0.67–0.96) |
| Sedrakyan et al. | Cisplatin-based chemotherapy Single-agent UFT | 12 7 (total = 7200) | 0.89 (0.82–0.96) 0.83 (0.73–0.95) |
| Bria et al. | Cisplatin-based chemotherapy | 12 (6494) | 0.93 (0.89–0.95) a |
| Hamada et al. b | UFT (single agent or combined with chemotherapy) | 6 (2003) | 0.74 (0.61–0.88) |
| Pignon et al. (LACE Collaborative Group) b | Cisplatin-based chemotherapy | 5 (4584) | 0.89 (0.82–0.96) |
| NSCLC Meta-analyses Collaborative Group b | Cisplatin-based chemotherapy Single-agent UFT | 17 (4406) 16 (3848) | 0.90 (0.82–0.98) 0.80 (0.71–0.90) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree