Introduction
A stroke is defined as ‘rapidly-developed clinical signs of focal or global disturbance of cerebral function, lasting more than 24 h or until earlier death, with no apparent non-vascular cause’ by the World Health Organization.1 A stroke is therefore distinguished from a transient ischaemic attack (TIA), which is defined similarly, except that the focal neurological symptoms must recovery fully within 24 h. The term ‘cerebrovascular accident’ should be avoided because of its negative connotations. Stroke carries with it a significant personal, community and economic burden. It is estimated that, in the USA alone, around 795 000 patients suffer a stroke each year at a direct and indirect financial cost of over US$70 billion.2 When considered separately from other cardiovascular diseases, stroke is the third leading cause of death in the Western world, and in the world as a whole it is the second commonest cause of death. It is also the leading cause of disability in survivors. Stroke occurs at all ages from birth onwards, but becomes increasingly common with increasing age and is a major cause of suffering in the elderly population.
The management of acute stroke is evolving and applied evidence from recent randomized controlled trials, along with better management of associated risk factors, has contributed to a relative reduction in age-adjusted morbidity and mortality from the condition in many countries. There now exists a good evidence base for hyperacute and acute interventions, and also for pharmacotherapy with the aim of secondary prevention. Acute stroke will, however, remain an important and common medical presentation as the proportion of the population aged over 65 years increases in many developed countries.
This chapter addresses the aetiology of stroke, the immediate management of the patient presenting with an acute stroke, the initiation of secondary prevention measures and the management of carotid artery stenosis. Intracerebral and subarachnoid haemorrhage are also discussed, as these conditions will also present to the geriatrician or stroke physician with the rapid onset of a focal neurological deficit.
Stroke Aetiology
It is important to recognize that the terms ‘stroke’ and ‘TIA’ describe the presentation of the patient and are not sufficient as a diagnosis on their own. This requires a description of the underlying pathology (haemorrhage or infarction), anatomy (vessel and territory involved) and aetiology (mechanism). Ischaemic stroke may occur as a result of a number of heterogeneous conditions. The major precipitant of cerebral ischaemia in the elderly population is atherosclerosis. Atheromatous plaque may occlude the artery at the site of buildup, but more commonly plaque rupture with superimposed thrombosis results in occlusion or embolization to a smaller, more distal artery, precipitating cerebral ischaemia. Smaller ‘lacunar’ infarcts may be caused by microatheroma of the deep penetrating arteries of the brain or by hyaline degenerative disease of the arterioles, which together are known as small-vessel disease. The pathogenesis of atherosclerosis is discussed in more detail later in this chapter. Alternatively, stroke may be caused by intracranial haemorrhage, cerebral venous thrombosis or cardiac embolism.
Cardiac embolism may arise from thrombus formation in the left atrium promoted by arrhythmia (especially atrial fibrillation or flutter), valvular heart disease or valve prosthesis or in myocardial infarction where mural thrombosis may develop on the injured myocardium. An inter-atrial or inter-ventricular septal defect (e.g. patent foramen ovale) may allow the passage of an embolus from the venous circulation to the left side of the heart, with the potential for subsequent paradoxical embolization to the cerebral vessels.
A number of other conditions may either cause ischaemic stroke or mimic its signs and symptoms and should be considered in the differential diagnosis. Information from history, examination and investigation pointing to an alternative cause should be pursued vigorously, as the appropriate and timely management of the underlying pathology may alter the chance of recurrent disease (Table 57.1).
Table 57.1 Classification of causes of stroke.
| Cerebral infarction |
Extracranial arterial embolism (internal carotid artery, aorta or vertebral artery)
|
Cardiac embolism
|
Paradoxical embolism
|
Trauma
|
Iatrogenic embolism
|
Intracranial arterial thrombosis
|
Vasospasm
|
Haemodynamic ischaemia
|
Cerebral venous thrombosis
|
| Intracranial haemorrhage |
Subarachnoid haemorrhage
|
| Intracerebral haemorrhage |
|
Stroke Mimics
Conditions that may mimic stroke but that are not of vascular origin include space-occupying lesions (e.g. subdural haematoma, tumour or abscess), hypoglycaemia, functional disorders and epilepsy, especially post-ictal paresis (Table 57.2).
Table 57.2 Differential diagnosis—stroke ‘mimics’.
Traumatic
|
Metabolic
|
Structural
|
Infective
|
| Functional |
Genetic disorders
|
Disorders of neural and neuromuscular function
|
Prognosis of Stroke
The mortality following first ischaemic stroke may be as high as 41% after 5 years,3 with vascular causes of death (stroke and myocardial infarction) most common during the first few weeks following the event.3 The risk of recurrent stroke is highest in this acute period. When patients are divided into groups depending on the aetiology of their stroke, those with significant large-artery (carotid) atherosclerosis are at highest risk of recurrence within the first month. Recurrence is least likely in those with small-vessel disease as a cause of their stroke.
Clinical Evaluation and Stroke Syndromes
Evaluation of the patient should begin with a detailed history. In the case of the aphasic, obtunded or confused patient, a witness history should be obtained from the ambulance staff, relative, friend or carer. It is important to establish the exact time of onset of symptoms, the progression or regression of a deficit over time and any associated features such as headache, loss of consciousness or possible seizure. Important details from past medical history will be the presence of other cardiovascular, neurological or haematological disease, medications taken, family history and ascertainment of risk factors. It is important to establish the patient’s baseline social function with respect to activities of daily living to plan future treatment, including resuscitation status, and also rehabilitation.
Examination should focus on establishing the nature and severity of the neurological deficit to aid in confirming the clinical diagnosis of stroke and establishing the likely site and size of cerebral lesion. Examination of other systems, especially the heart, may give clues to an underlying cause or prompt further investigation.
The severity of neurological deficit should be assessed using a formal tool such as the National Institutes for Health (NIH) Stroke Severity (NIHSS) Score.4 This examination should be performed at the time of presentation and grades the patient response in a number of domains, which comprise level of consciousness, gaze and vision, motor power, ataxia, language and speech and extinction and inattention, using specific validated techniques of examination. Higher scores are associated with more profound deficit and worse outcome, although the prominence of language domains in the score may underestimate the severity of the impact of right hemispheric syndromes. The NIHSS score is used to select patients for thrombolysis (see below) and monitor their early progress. Other scales, including the Barthel Index5 and modified Rankin score6 (Tables 57.3 and 57.4), grade disability and handicap and are used to guide rehabilitation and measure the progress or outcome of treatment.
Table 57.3 The Barthel Index.5.
| Bowels | |
| 0 | Incontinent (or needs to be given enemata) |
| 1 | Occasional accident (once per week) |
| 2 | Continent |
| Bladder | |
| 0 | Incontinent or catheterized and unable to manage |
| 1 | Occasional accident (maximum once per 24 h) |
| 2 | Continent (for over 7 days) |
| Grooming | |
| 0 | Needs help with personal care |
| 1 | Independent face/hair/teeth/shaving (implements provided) |
| Toilet use | |
| 0 | Dependent |
| 1 | Needs some help, but can do something alone |
| 2 | Independent (on and off, dressing, wiping) |
| Feeding | |
| 0 | Unable |
| 1 | Needs help cutting, spreading butter, etc. |
| 2 | Independent (food provided within reach) |
| Transfer | |
| 0 | Unable—no sitting balance |
| 1 | Major help (one or two people, physical), can sit |
| 2 | Minor help (verbal or physical) |
| 3 | Independent |
| Mobility | |
| 0 | Immobile |
| 1 | Wheel chair independent including corners, etc. |
| 2 | Walks with help of one person (verbal or physical) |
| 3 | Independent (but may use any aid, e.g. stick) |
| Dressing | |
| 0 | Dependent |
| 1 | Needs help, but can do about half unaided |
| 2 | Independent (including buttons, zips, laces, etc.) |
| Stairs | |
| 0 | Unable |
| 1 | Needs help (verbal, physical, carrying aid) |
| 2 | Independent up and down |
| Bathing | |
| 0 | Dependent |
| 1 | Independent (or in shower) |
| Total | (0–20) |
Table 57.4 The Modified Rankin Score.6.
| Gradea | Description |
| 0 | No symptoms at all |
| 1 | No significant disability despite symptoms: able to carry out all usual duties and activities |
| 2 | Slight disability: unable to carry out all previous activities but able to look after own affairs without assistance |
| 3 | Moderate disability: requiring some help, but able to walk without assistance |
| 4 | Moderately severe disability: unable to walk without assistance and unable to attend to own bodily needs without assistance |
| 5 | Severe disability: bedridden, incontinent and requiring constant nursing care and attention |
aIn clinical trials, a grade of 6 is often added to record patients who have died.
Identifying the likely size and location of the arterial territory involved from the symptoms and signs is an important aid to determining the mechanism and prognosis of stroke. The commonest arterial syndromes are described in the next section. Occlusion and rupture of vessels (i.e. infarction or haemorrhage) result in similar syndromes, which are indistinguishable clinically. However, in the next section, the description concentrates on ischaemic stroke for simplicity.
Total Middle Cerebral Artery (MCA) Syndromes
Occlusion of the trunk of the middle cerebral artery (MCA) carries the gravest prognosis and may cause infarction of a large area of brain tissue supplied by its superficial cortical branches and the deep lenticular branches supplying basal ganglia and internal capsular white matter. There is cortical dysfunction (such as a speech or spatial disorder depending on the hemisphere involved), motor and/or sensory deficit of the face and arm or arm and leg and homonymous visual field defect. There may also be a decreased level of consciousness secondary to oedema and brainstem compression, which usually develops over the course of the first 24–48 h after onset. Total MCA territory infarction is frequently fatal, with a 1 year mortality of up to 40%, depending partly on the care provided.7
Partial MCA Syndromes
Branch occlusions of the MCA are heterogeneous in their presentation. If the upper division of the MCA is affected, then typically there is no visual field defect. If the lower division of the MCA is affected, then typically there is a reduced motor or sensory component to symptoms and signs. Branch occlusion is more commonly caused by embolism than local thrombus. Prognosis is variable, with a 1 year mortality of up to 16%, but 55% of patients are independent at 1 year.7
Lacunar Syndromes
Occlusion of small perforating arterioles supplying the basal ganglia, external or internal capsule and the pons results in small infarcts known as lacunes. These have been defined on brain imaging as deep subcortical infarcts less than 15 mm in maximum diameter. They are caused by occlusion of the smaller perforating arteries either secondary to microatheroma at the vessel origin or secondary to more distal lipohyalinosis in which degenerative pathological changes occur in the tunica media and adventitia of the vessels. These two pathologies are difficult to distinguish clinically or radiologically and together are often known as small-vessel disease. This term is also frequently used by radiologists to describe the appearances of patchy or confluent low attenuation on computed tomographic (CT) or high signal on T2 and fluid-attenuated inversion–recovery (FLAIR) magnetic resonance imaging (MRI) scans, in the periventricular deep white matter. These changes are also known as leukoaraiosis and are thought to be another manifestation of lipohyalinosis, as are microhaemorrhages seen on gradient echo (T2*) MRI. Leukoaraiosis, lacunes and microhaemorrhages are independently associated with cognitive impairment. Hypertension is a major risk factor for lacunar infarction, leukoaraiosis and microhaemorrhages. The last are also associated with cerebral amyloid angiopathy.
Four common presentations of lacunar infarction, known as lacunar syndromes, are described. Pure motor stroke presents with unilateral face, arm and leg weakness. The weakness may not involve all three sites, but should involve at least two contiguous sites, that is, face and arm or arm and leg. Pure sensory stroke gives rise to hemisensory loss on the contralateral side. A larger infarct may give rise to a mixed motor/sensory stroke, but the characteristic feature of lacunar infarction remains, which is that there is no disturbance of higher (cortical) function and no visual field deficit. The fourth common type of lacunar stroke, ataxic hemiparesis, describes limb ataxia in combination with a hemiparesis and/or dysarthria. These syndromes arise from lacunar infarction (or occasionally small haemorrhages) in either the internal capsule or the pons and imaging is required to distinguish these sites. Numerous rarer lacunar syndromes are described, including unilateral chorea and hemiballismus from lacunes in the basal ganglia.
Lacunar stroke carries a good prognosis for survival, with a 1 year mortality of about 10%, but 50% of survivors remain dependent.7
Posterior Circulation Syndromes
Vertebrobasilar (or posterior) circulation syndromes result from ischaemia of the brainstem, cerebellum and/or occipital lobes, and therefore the symptoms experienced are varied depending on the vessels and territories affected. Brainstem infarction from basilar artery or perforating vessel occlusion can result in cranial nerve dysfunction, eye movement disorders and/or nystagmus, with or without bilateral motor and/or sensory deficit. Pontine infarction secondary to basilar artery thrombosis may cause the ‘locked-in’ syndrome. This produces quadriplegia, but with preservation of eye movements and blinking which may facilitate communication. Occlusion of one of the posterior cerebral arteries results in hemianopia, while occlusion of both posterior cerebral arteries results in cortical blindness. In some patients, the posterior cerebral artery supplies the median temporal lobe, in which case the visual field defect may be accompanied by mild or, if bilateral, severe memory impairment (amnesia). In about 5% of individuals, the posterior cerebral artery is supplied by the ipsilateral internal carotid artery via a dominant posterior communicating artery. In such cases, occipital infarction and hemianopia may result from carotid disease.
Cerebellar infarction may result in gait ataxia, but not necessarily any other cerebellar signs. Occlusion of the posterior inferior cerebellar artery (PICA) may produce the characteristic lateral medullary (Wallenberg) syndrome. This comprises vestibular dysfunction, ataxia, ipsilateral loss of pain and temperature sensation from the face, ipsilateral Horner syndrome and contralateral loss of pain and temperature sensation in the arm and leg. Dysarthria and dysphagia are not specific for brainstem lesions and can occur with any motor syndrome.
In contrast to anterior circulation syndromes, thrombosis of the basilar artery may be responsible for a higher proportion of strokes than embolism from a distant site. The 1 year mortality for posterior circulation infarction is 19%, with 62% of patients independent at 1 year.7
Thalamic Syndromes
The blood supply of the thalamus is carried by branches of the vertebrobasilar system and also the tuberothalamic artery that arises from the posterior communicating artery. Thalamic nuclei participate in sensory and motor function, language, cognitive function, alertness, memory, mood and motivation.8 Hence the clinical syndrome that results from thalamic infarction depends on the specific nuclei involved. Unilateral infarction may cause memory loss or confusion, language disturbance when the dominant hemisphere is affected and spatial deficit when the non-dominant hemisphere is affected. The thalamic syndrome describes the combination of contralateral sensory loss and hemiparesis associated with the development of distressing post-stroke pain in the affected limbs. Bilateral infarction of the thalamus may result in coma with subsequent severe amnesia.8
Prehospital Care
In many countries where hyperacute stroke treatments such as thrombolysis are available, the spotlight of public health has fallen on the early recognition of stroke by both the general public and allied health professionals.
Validated clinical tools such as the FAST (‘Face, Arm, Speech Test’) screening test9 allow paramedics and the public to identify symptoms of stroke and can allow triage to the correct facility with early warning of the patient’s arrival. FAST instructs the user to evaluate facial movement, upper limb power and speech to identify a neurological deficit and paramedic findings on examination correlate well with subsequent examination findings by a trained physician. It may, however, be less sensitive for the detection of posterior circulation events,9 which has led to the development of the ROSIER (‘Recognition of Stroke in the Emergency Room’) Scale, which incorporated a measure of hemianopia and may be more suitable for use by physicians in the emergency department. Prospective validation of this scale in 173 patients referred with suspicion of stroke showed sensitivity of 93% (95% CI, 89–97%) and specificity of 83% (95% CI, 77–89%) for the diagnosis of stroke.10
A number of other, similar, screening tools in Melbourne (MASS), Cincinnati (CPSS) and Los Angeles (LAPSS) have also been described in the literature.
Initial Investigations and Imaging
The investigation of stroke and transient ischaemic attack are similar and are designed to establish the pathology, anatomy and mechanisms of symptoms and plan treatment. All patients should have urea, electrolytes, creatinine, full blood count, clotting screen, serum glucose and erythrocyte sedimentation rate carried out when they are first seen. A fasting glucose and fasting cholesterol should be done as soon as possible to exclude hypercholesterolaemia and diabetes. Electrocardiography will confirm the clinical assessment of heart rhythm or document evidence of ischaemia or acute myocardial infarction. If symptoms or clinical signs suggest respiratory involvement, such as heart failure or aspiration pneumonia, a chest X-ray should be added to these investigations. Further biochemical investigation of rarer causes of ischaemic stroke or stroke mimics will be directed by the clinical picture.
The immediate concern in acute stroke is the rapid differentiation of ischaemic from haemorrhagic stroke, which is most quickly and reliably demonstrated by non-contrast CT imaging. CT is sensitive and specific for haemorrhage within the first 8 days after stroke,11 after which time reliability of detection decreases. MRI as an alternative is discussed below. All patients admitted with suspected stroke should undergo cranial imaging as soon as possible after admission and definitely within 24 h.11, 12 An immediate emergency scan is required for patients being considered for thrombolytic therapy or for those at risk of intracerebral haemorrhage by virtue of their presentation, including reduced conscious level, sudden onset of severe headache, a history of trauma, known coagulopathy or therapeutic anticoagulation.
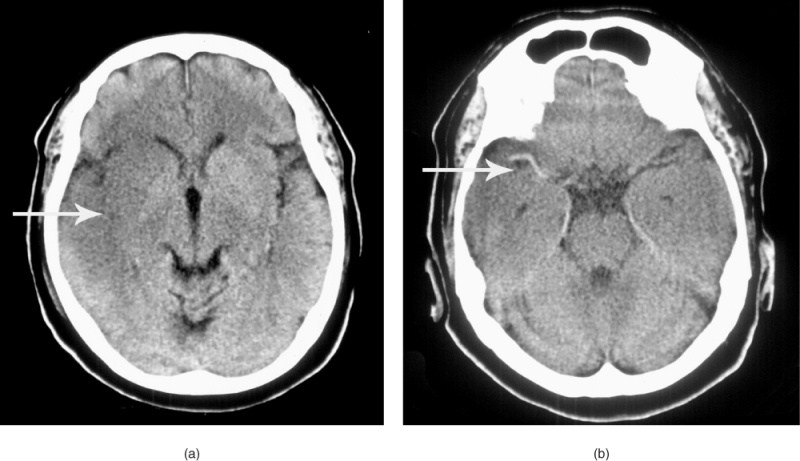
Early signs of ischaemic stroke on CT include hyperdensity of a cerebral artery (showing thrombus in situ), slight hypodensity of the brain parenchyma leading to loss of the clear definition of the insular ribbon and lentiform nucleus, sulcal effacement reflecting tissue swelling and mass effect from more pronounced oedema (Figure 57.1).13 A large hypodense area seen on CT reflects a poor prognosis and is sometimes used as a contraindication to thrombolysis because the risk of subsequent haemorrhage may be increased. This is because hypodensity reflects increased water content of the tissue and suggests both established infarction and the breakdown of the blood–brain barrier in this region. If the hypodensity is clearly delineated, this indicates that the infarction is well established and not hyperacute.
Figure 57.1 (a) Early changes of right MCA territory infarction on CT scan. (b) MCA hyperdensity in the same patient.
CT angiography (CTA) may be carried in the same session as non-contrast CT to visualize acute intracranial vessel occlusion, and also extravascular and intravascular stenosis or dissection. Acute CT perfusion imaging may also have a role in the future, allowing assessment of the extent of hypoperfusion in an arterial territory.
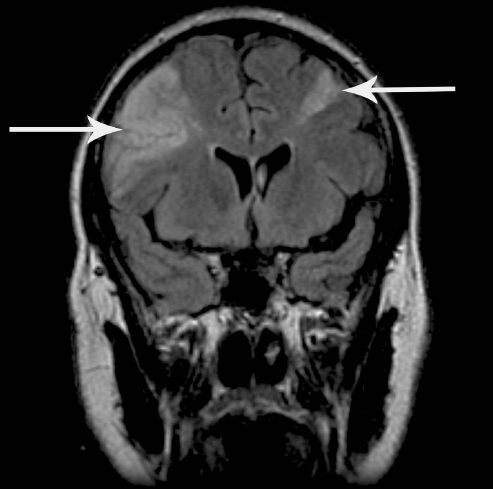
CT has the limitation that it is normal within the first few hours after onset in 30–40% of patients.13 MRI is much better at detecting early changes of infarction and small infarcts, especially in the posterior fossa. MRI is also the technique of choice to identify haemorrhage if the scan is performed more than 8 days after onset. Several MRI sequences can be combined in one examination, termed ‘multi-modal MRI’, to produce a variety of clinically useful images. T2 sequences provide good anatomical definition and show established infarction well, but are not very sensitive to early changes. Diffusion-weighted imaging (DWI) is much more sensitive than CT to the early and chronic changes of infarction. DWI becomes abnormal within a few minutes of onset of infarction and shows areas of restricted extracellular movement of water molecules, secondary to ischaemic membrane pump failure and cell swelling from excessive intracellular water, as bright areas. When the infarction is complete and cell lysis has occurred, usually between 7 and 14 days after onset, diffusion of water is facilitated and DWI will then show infarcts as dark areas. However, DWI may also show older infarcts as bright areas because of the phenomenon of ‘T2 shine through’. The same MR sequences are therefore used to generate apparent diffusion coefficient (ADC) maps, which show acute infarction as dark and older infarcts as brighter areas.14 Gradient echo imaging (GRE or T2*) is very sensitive to new and old haemorrhage. FLAIR imaging is highly sensitive to areas of gliosis secondary to established infarction and periventricular small-vessel disease (Figure 57.2). Magnetic resonance angiography (MRA) provides an alternative to CTA for vascular imaging. MR perfusion imaging can show areas of impaired blood flow secondary to arterial occlusion or severe stenosis.
Thus MRI has considerable advantages over CT, in addition to avoiding ionizing radiation. If available in the emergency situation, MRI with DWI can replace CT or may be used as an additional investigation. It should be considered a routine investigation in stroke patients in whom CT has failed to establish the diagnosis and is the investigation of choice after TIA. Limitations of MRI include patient tolerability, increased cost compared with CT and the requirement to exclude patients with metallic foreign bodies or implants.
In-Hospital Care
As with any acutely unwell patient presenting to hospital, the standard initial assessment and resuscitation of a patient with suspected stroke begin with attention to the airway, breathing and circulation.
Airway
The airway in a stroke patient may be obstructed secondary to a reduced level of consciousness or a buildup of secretions that cannot be effectively swallowed. Simple airway manoeuvres—for example, chin lift or jaw thrust—may be sufficient to clear the airway, or the use of an airway adjunct may be required. Patients with ongoing airway problems may warrant consideration of the involvement of an anaesthetist as an airway specialist.
Breathing
Effective breathing, and therefore effective oxygenation of the blood and brain, may be compromised by a number of mechanisms. A stroke affecting the respiratory centres of the brainstem may cause apnoea or bradypnoea. Problems with swallowing may precipitate aspiration pneumonia. In addition, hypoxaemia may arise through an associated condition such as heart failure or pulmonary embolism. Initial management is directed at treatment of the underlying cause, although guidelines recommend the application of inhaled oxygen only where a new desaturation below 92–95% exists on pulse oximetry.12
Circulation
In ascertaining the heart rhythm and blood pressure, some of the risk factors for stroke are being assessed. A finding of an elevated blood pressure is almost universal in patients within 48 h of stroke onset. This may occur either secondary to the stroke itself or reflect pre-existing hypertension. The management of high blood pressure in acute stroke is discussed later in this chapter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree